Reducing Matrix Impact on Trace Analysis with GC–MS: A Simple Capillary Flow Technology "Tee" for Improved Backflush Efficiency
The Application Notebook
Capillary flow technology (CFT) devices are microfluidic components that extend capillary GC capabilities through simple and robust connections between pressure/flow modules and columns. One of the most powerful and simple is the CFT Tee. This is especially useful in GC–MS analysis providing (1) rapid column and inlet maintenance without MSD venting and (2) the capability of rapidly removing late eluting interferences from the column by forcing their retreat into the injection port through "backflushing". Removing these interferences improves column and detector longevity and analytical integrity. Backflushing is very valuable for trace GC–MS analysis in samples from complex matrices like soil, foods or tissues. The CFT Tee uses pressure-pulsed injections and constant flow mode with minimal loss in the MS signal. This approach will be useful to all GC–MS users who want to improve their instrument uptime.
Harry Prest, Agilent Technologies Inc., Santa Clara, California, USA.
Capillary flow technology (CFT) devices are microfluidic components that extend capillary GC capabilities through simple and robust connections between pressure/flow modules and columns. One of the most powerful and simple is the CFT Tee. This is especially useful in GC–MS analysis providing (1) rapid column and inlet maintenance without MSD venting and (2) the capability of rapidly removing late eluting interferences from the column by forcing their retreat into the injection port through "backflushing". Removing these interferences improves column and detector longevity and analytical integrity. Backflushing is very valuable for trace GC–MS analysis in samples from complex matrices like soil, foods or tissues. The CFT Tee uses pressure-pulsed injections and constant flow mode with minimal loss in the MS signal. This approach will be useful to all GC–MS users who want to improve their instrument uptime.
Experimental Arrangement
Schematically the arrangement is shown in Figure 1. A CFT Tee splits a typical 30 m column so the configuration is simple: one 15 m column connects the split/splitless injection port to the Tee; another 15 m column from the Tee to the Agilent 5975C MSD; and the rear injection port connects to the Tee. The pressure/flow at the Tee is controlled by the rear injection port Electronic Pressure Control (EPC) module. All flow calculations were made automatically by the 7890A GC EPC. Pulsed splitless injections under constant flow mode were made of a 6 component mixture to illustrate backflushing and selectivity against late eluters.
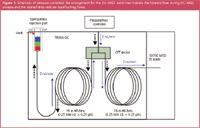
Figure 1
Results and Conclusions
The upper panel in Figure 2 shows the reconstructed total ion chromatogram (RTIC) of the 6 component standard. The last analyte is the third peak and the fourth peak the beginning of late eluting interferences. The middle panel is the RTIC of the same standard with backflushing beginning at 10.1 min (a) when the last analyte passes the Tee, enters the second column and roughly 0.4 min later enters the MSD. Backflushing begins at time (a) when the first 15 m column flow is dropped so late eluters begin to retreat in the first column while the final analyte elutes. At time (b) the second 15 m column flow is increased and the late eluters never enter the MSD but are pushed back through the first column into the injection port. The lower panel is a solvent blank run, which shows no carryover. This approach to backflushing is more rapid than "baking off" late eluters or backflushing an entire column.

Figure 2
Column maintenance is similarly simple: cool the port, raise the pressure at the Tee and cutback the column or service the liner and septum. No air will enter the MSD.
For more details, see reference 3.
References
(Literature Library at www.chem.agilent.com)
1. The 5975C Series GC/MSD (5989-7827EN)
2. GC/MS Analysis of PCBs in Waste Oil using the backflush capability of the Agilent QuickSwap Accessory (5989-7601EN)
3. Capillary Flow Technology for GC/MS: a simple Tee configuration for analysis at trace concentrations with rapid backflushing for matrix elimination (5989-8664EN)

Agilent Technologies Inc.
Hewlett-Packard Strasse 8, PO Box 1280
D-76337 Waldbronn, Germany
tel. +49 7243 602 107 fax +49 7243 602 501
Website: www.agilent.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)













