Increasing LC–MS–MS Sensitivity with Luna HILIC
The Application Notebook
The analysis of polar compounds in support of clinical and preclinical pharmacokinetic studies requires an analytical methodology capable of achieving ultra-low detection and quantification limits. The high sensitivity afforded by coupling HPLC with tandem mass spectrometry (MS–MS) has made it the technique of choice in this environment, but it is subject to the following limitations when reversed-phase liquid chromatography (RPLC) is used
Carl Sanchez, Monika M. Kansal, Art Dixon, Philip J. Koerner and Terrell Mathews, Phenomenex Inc., Torrance, California, USA.
Introduction
The analysis of polar compounds in support of clinical and preclinical pharmacokinetic studies requires an analytical methodology capable of achieving ultra-low detection and quantification limits. The high sensitivity afforded by coupling HPLC with tandem mass spectrometry (MS–MS) has made it the technique of choice in this environment, but it is subject to the following limitations when reversed-phase liquid chromatography (RPLC) is used:
1. Polar compound elution in a highly aqueous mobile phase: Gas phase ion generation is facilitated with more volatile solvents;1 therefore, the high water content used for RPLC retention of polar compounds decreases desolvation efficiency. In addition the highly aqueous mobile phase required for retention of polar compounds increases solvent surface tension, which decreases ESI spray stability.2
2. Polar compound elution in the primary ion suppression region: Poorly retained compounds in RPLC increase the likelihood of analyte compounds eluting with the many endogenous compounds present in bioanalytical samples.These endogenous compounds compete for charge in the source and can suppress analyte ionization, reducing sensitivity and degrading LOD and LOQ. It is critical that a robust bioanalytical method separate analytes from the ion suppression region.3
Luna HILIC columns allow for improved sensitivity in the analysis of complex bioanalytical samples by creating more favorable conditions for polar compound retention and ionization.
On the Luna HILIC column, atenolol elutes in a high organic volatile mobile phase, which facilitates analyte desolvation and results in enhanced LC–MS–MS sensitivity. Polar metabolites are weakly retained under RPLC conditions and elute near the solvent front, often in the "primary ion suppression region". Under HILIC chromatographic conditions, where polar analytes are retained using high organic mobile phase, Luna HILIC strongly retains polar compounds that are otherwise weakly retained in RPLC.
Conclusion
The Luna HILIC column retains and elutes hydrophilic/polar compounds in highly organic mobile phase conditions, which improve analyte desolvation efficiency and ESI spray stability. The combination of these two improvements in the MS interface will often provide improved polar analyte detection and quantitation.

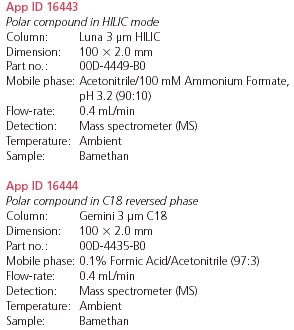
Figure 1
In HILIC mode, the analyte elution in the primary ion suppression region is avoided as a result of the mode's increased retention of hydrophilic compounds. This increased retention for polar compounds regularly results in less interference from endogenous sample compounds, improved sensitivity and accuracy, and reduced LOD and LOQ. The Luna HILIC column meets the requirements of a robust bioanalytical method by eluting analytes outside the primary ion suppression region.

Figure 2
It has been shown here that Luna HILIC columns can improve LC–MS–MS sensitivity of polar compounds in biological matrices in support of clinical and preclinical pharmacokinetic studies where ultra-low detection and quantification limits are highly desirable.
References
1. N.B. Cech and C.G. Enke, Mass Spectrom. Rev., 20, 362–387 (2001).
2. P.J. Kerbarle, Mass. Spectrom., 35, 804–817 (2000).
3. B.K. Matuszewski, M.L. Constanzer and C.M. Chavez-Eng, Anal. Chem., 75, 3019–3030 (2003).
Luna and Gemini are registered trademarks of Phenomenex Inc.

Phenomenex Inc.
411 Madrid Avenue, Torrance, California 90501, USA
tel. +1 310 212 0555 fax +1 310 328 7768
E-mail: info@phenomenex.com
Website: www.phenomenex.com

Characterizing Polyamides Using Reversed-Phase Liquid Chromatography
May 5th 2025Polyamides can be difficult to characterize, despite their use in various aspects of everyday life. Vrije Universiteit Amsterdam researchers hoped to address this using a reversed-phase liquid chromatography (RPLC)-based approach.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)













