Recent Advances in Solid-Phase Microextraction, Part 1: New Tricks for an Old Dog
LCGC Europe
Solid-phase microextraction (SPME) was introduced nearly 30 years ago and since that time has matured into a widely used tool in the arsenal of sample preparation techniques. Simultaneously, it has spawned a host of related techniques where sorbent coatings are placed on stir bars, magnetic particles, vial walls, and so on. Over the past few years, several advances in SPME have been developed, including increasing the sorbent surface area available for extraction, accommodating direct analysis by mass spectrometry (MS), and sorbent overcoating to resist fouling by sugars, lipids, and other macromolecules present in some sample types. These advances are discussed in this month’s instalment. The use of SPME for microsampling of biological systems, so-called bio-SPME, will be the focus of Part 2.
Solid-phase microextraction (SPME) was introduced nearly 30 years ago and since that time has matured into a widely used tool in the arsenal of sample preparation techniques. Simultaneously, it has spawned a host of related techniques where sorbent coatings are placed on stir bars, magnetic particles, vial walls, and so on. Over the past few years, several advances in SPME have been developed, including increasing the sorbent surface area available for extraction, accommodating direct analysis by mass spectrometry (MS), and sorbent overcoating to resist fouling by sugars, lipids, and other macromolecules present in some sample types. These advances are discussed in this month’s instalment. The use of SPME for microsampling of biological systems, so-called bio-SPME, will be the focus of Part 2.
The evolution of chemical extractions using sorptive phases follows an interesting history. It likely started with the column chromatography cleanup procedures that we all performed in our sophomore organic chemistry classes and that are widely used in the organic discipline. This evolution eventually led to solid-phase extraction (SPE), which was developed and popularized beginning in the 1970s. But the big breakthrough was the advent of solid-phase microextraction (SPME). Perhaps what is most notable is that as SPME gained acceptance, a plethora of techniques sprouted where the researchers seemingly attempted to place a stationary phase on any surface that could potentially be exposed to a sample-inside needles and capillaries, on vial surfaces and lids, on stir bars, and so on. Since the patent protection on SPME was lifted, several new advances have been noted. In this instalment, we provide an overview of some of these major advances related to the traditional practice of SPME.
Applications
A series of three critical reviews explored the application of SPME in environmental (1), food (2), and clinical (3) analysis. Environmental samples like soils, sediments, and wastewater were discussed and thinâfilm and cold-fibre SPME approaches were emphasized (1). In vivo SPME of living animals and plants was reviewed for environmental and ecological studies (4). Particular interest on quantification methods is noted. A discussion of SPME used for food analysis includes emphasis of new phases, calibration studies, and discusses the determination of contaminants and food quality (2). Another review of food applications of SPME looked at volatile, semivolatile, and nonvolatile components from a diverse set of food samples (5). Fibres overcoated with polydimethylsiloxane (PDMS) did not show the matrix interferences common in food analysis using direct immersion SPME (6). We will not discuss the bioanalytical and clinical analysis review (3) in this instalment of “Sample Preparation Perspectives” because it will be the topic of part 2 in this series.
Commercial Advances
Only recently have multiple SPME fibres been able to be desorbed automatically in a single gas chromatography (GC) sequence without manual intervention. In evaluating the Gerstel MultiâFiber Exchange (MFX) system, modifications to the cap and potential cooling minimized sample storage stability, defined as less than 10% sample loss (7). Short-term exposure and timeâweighted average data were obtained and a new diffusive fibre holder was investigated.
One new configuration for SPME sampling is the Custodian SPME syringe marketed by PerkinElmer. Designed for field sampling, the syringe features a ballpoint penâstyle mechanism for situations where analyst dexterity may be limited.
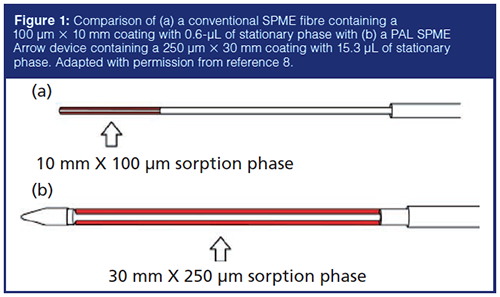
Another design is the PAL (Prep and Load) SPME Arrow (8,9). Key features of the device are a stainless steel fibre designed to provide a greatly enhanced sample capacity, as illustrated in Figure 1. Another stated advantage of the SPME Arrow is that the design minimizes coring of the GC injector septum, allowing a greater number of injections before septa must be replaced. Although improved sample capacity is observed, the sorption–desorption times are significantly increased if this greater capacity is to be realized. But the sensitivities gained with the SPME Arrow device fall between conventional SPME and stirâbar sorptive extraction (SBSE).
In-Tube SPME
Perhaps the simplest alternate form of SPME is in-tube SPME. In this design, a section of an openâtubular (capillary) GC column is attached to a syringe and draw–eject cycles are used for solute sampling and desorption. This approach is most widely used with subsequent liquid chromatography (LC) analysis and is often performed in an online SPME-LC mode. One recent review (10) reported that most accounts of in-tube SPME used 250âµm i.d. columns, though diameters ranging from 10 to 750 µm have been used, with lengths ranging from a few centimetres to 80 cm. There was no correlation between column diameter and length, though if in-tube SPME is combined online with LC, it must be appropriate for the separation column. Obviously, the larger the surface area, the greater the sample capacity. Solvent desorption, rather than the thermal desorption used in traditional SPME, alleviates concerns with slow desorption steps. They also report that typical siloxane stationary phases are used for extraction of environmental, food, and industrial products, while carbon-based phases like polyethylene glycol and molecular sieves are used in bioanalysis, as well as with environmental and food products. Others, such as Fernandez-Amado and colleagues (11), assert that the type of capillary and coating are the most influential parameters for in-tube SPME. They report that, of the published applications using the technique, 38.6% involved environmental analysis, 37.3% were biomedical, and food applications accounted for 21.7% of articles.
Other Configurations
Fused-silica-lined bottles provided sample stability for the collection and storage of a headspace subsample with repetitive analysis using fibreâbased SPME (12). Samples could be held in the bottle for up to 30 days with no sample loss, and the 400-mL bottle volume allowed multiple sampling via SPME.
A passive sampler made from an SPME device isolated hydrophobic organic compounds in natural waters for subsequent measurement and modelling of bioaccumulation by benthic invertebrates (13).
Sorptive Phase Development
Hou, Wang, and Guo (14) classified current SPME phases into inorganic coatings (metal–organic frameworks, carbon nanotubes, graphene, and metal and metal oxides), organic polymer coatings (polymeric ionic liquids and molecularly imprinted polymers), and others (aptamerâfunctionalized materials, nanofibres) and discussed their use. Carbon nanotubes modified with PDMS and used as a sol-gel are shown to have several advantages for SPME including high porosity, a long lifetime, and high thermal stability (15). Wide linear ranges and low detection limits are obtained with these devices. Microwave-induced plasma treatment of stainless steel fibres led to improved adherence of multiwalled carbon nanotubes (16). In general, carbon nanotubes possess high surface area and large conjugated π-cloud systems, both of which lead to superior adsorption capability. This inexpensive method provided high thermal stability, solution resistance, and high surface area to the SPME fibre. As an example of metal–organic frameworks, an aluminium-based metal–organic framework with a polymethacrylate monolith provided efficient isolation of penicillins in milk samples using in-tube SPME (17). Ansari and Karimi reviewed the application of molecular-imprinted polymers (MIP) for drug analysis in the coated, in-tube, monolithic, solâgel, and membrane SPME modes (18). The low cost, high porosity, reproducibility, and increased sensitivity are touted as advantages of MIPs. Another review of MIPâSPME focused on applications (19). In another study, hydrofluoric acid was used to etch the surface of a stainless steel wire and increase its surface area (20). Then the wire was coated with an ionic liquid as the sorptive phase and, after optimizing conditions, used for the extraction of aqueous alkylphenols with enrichment factors 6.5 to 16.8 times greater than that with a commercial polyacrylate fibre with an 85-µm film. Glass-fibre filters coated with PDMS in the capillary microextraction mode were found to be 30-fold more sensitive than dynamic SPME for isolating methamphetamine vapours associated with forensic samples (21).
Conclusion
Despite its longevity in the sample preparation world, SPME is seeing a tremendous growth spurt. Recent advances and those expected in the foreseeable future centre primarily on the development of novel sorptive phases and formats that allow for the analysis of emerging systems, such as sampling living systems or harsh environments.
References
- E.A. Souza-Silva, R. Jiang, A. Rodriguez-Lafuente, E. Gionfriddo, and J. Pawliszyn, TrAC Trend Anal. Chem.71, 224–235 (2015).
- E.A. Souza-Silva, E. Gionfriddo, and J. Pawliszyn, TrAC Trend Anal. Chem.71, 236–248 (2015).
- EA. Souza-Silva, N. Reyes-Garces, G.A. Gomez-Rios, E. Boyaci, B. Bojko, and J. Pawliszyn, TrAC Trend Anal. Chem.71, 249–264 (2015).
- J. Xu, G. Chen, S. Huang, J. Qiu, R. Jiang, F. Zhu, and G. Ouyang, TrAC Trend Anal. Chem.85, 26–35 (2016).
- C.-H. Xu, G.-S. Chen, Z.-H. Xiong, Y.-X. Fan, X.-C. Wang, and Y. Liu, TrAC Trend Anal. Chem.80, 12–29 (2016).
- A. Naccarato and J. Pawliszyn, Food Chem.206, 67–73 (2016).
- G.A. Gomez-Rios, N. Reyes-Garces, and J. Pawliszyn, J. Sep. Sci.38, 3560–3567 (2015).
- A. Kremser, M.A. Jochmann, and T.C. Schmidt, Anal. Bioanal. Chem.408, 943–952 (2016).
- A. Helin, T. Ronkko, J. Parshintsev, K. Hartonen, B. Schilling, T. Laubli, and M.-L. Riekkola, J. Chromatogr. A1426, 56–63 (2015).
- P. Serra-Mora, Y. Moliner-Martinez, C. Molins-Legua, R.HerraezâHernandez, J. Verdu-Andres, and P. Campins-Falco, Comp. Anal. Chem.76, 427–461 (2017).
- M. Fernandez-Amado, M.C. Prieto-Blanco, P. Lopez-Mahia, S. MuniateguiâLorenzo, and D. Prada-Rodriguez, Anal. Chim. Acta 906, 41–57 (2016).
- C.A. Harvey, J.C. Carter, J.R. Ertel, C.T. Alviso, S.C. Chinn, and R.S. Maxwell, J. Chromatogr. A1401, 1–8 (2015).
- K.A. Maruya, W. Lao, D. Tsukada, and D.W. Diehl, Chemosphere137, 192–197 (2015).
- X. Hou, L. Wang, and Y. Guo, Chem. Lett.46, 1444–1455 (2017).
- A. Amiri and F. Ghaemi, Microchim. Acta184, 297–305 (2017).
- Z. Tang, Y. Liu, and Y. Duan, J. Chromatogr. A1425, 34–41 (2015).
- S. Lirio, W.-L. Liu, C.-L. Lin, C.-H. Lin, and H.-S. Huang, J. Chromatogr. A1428, 236–245 (2016).
- S. Ansari and M. Karimi, Talanta164, 612–625 (2017).
- A. Sarafriz-Yazdi and N. Razavi, TrAc Trend Anal. Chem.73, 81–90 (2015).
- M. Cui, J. Qiu, Z. Li, M. He, M. Jin, J. Kim, M. Quinto, and D. Li, Talanta132, 564–571 (2015).
- M.V. Nair and G.S. McKelly, Forensic Sci. Intl.268, 131–138 (2016).
“Sample Prep Perspectives” editor Douglas E. Raynie is a Department Head and Associate Professor at South Dakota State University, USA. His research interests include green chemistry, alternative solvents, sample preparation, high-resolution chromatography, and bioprocessing in supercritical fluids. He earned his Ph.D. in 1990 at Brigham Young University under the direction of Milton L. Lee. Raynie is a member of LCGC’s editorial advisory board. Direct correspondence about this column via e-mail to LCGCedit@ubm.com

Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.
University of Oklahoma and UC Davis Researchers Probe Lipidomic Profiles with RP-LC–HRMS/MS
May 6th 2025A joint study between the University of Oklahoma Health Sciences Center (Oklahoma City, Oklahoma) and the UC Davis West Coast Metabolomics Center (Davis, California) identified differentially regulated lipids in type 2 diabetes (T2D) and obesity through the application of reversed-phase liquid chromatography-accurate mass tandem mass spectrometry (RP-LC-accurate MS/MS).
Automated Sample Preparation (ISO 20122) for MOSH/MOAH in Seasoning Oils
May 6th 2025This work presents an Automated Sample Preparation procedure for MOSH/MOAH analysis of Seasoning Oils. We compare results from a manual epoxidation procedure compliant with DIN 16995 with results based on fully automated sample preparation (epoxidation and saponification) compliant with ISO 20122. In both cases, online clean-up via activated aluminum oxide (AlOx) are used to remove interfering n-alkanes from the MOSH fraction during the HPLC run. Automated data evaluation using a dedicated software (GERSTEL ChroMOH) is presented.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)