Rapid, Ultra-High Efficiency USP Assay for Ibuprofen Using Kinetex 2.6 µm XB-C18 Core-shell HPLC/UHPLC Column
Phenomenex Application Note
Philip J. Koerner, Deborah Jarrett and Jeff Layne, Phenomenex Inc., Torrance, California, USA.
Application of core-shell technology to the USP assay for ibuprofen to reduce analysis time and increase sample throughput and productivity, while improving the sensitivity of the assay for the determination of ibuprofen related compound C, a known degradation product with adverse health effects.
Introduction
The introduction of Kinetex core-shell columns has brought dramatic benefits to chromatographers. The ability to obtain ultra-high chromatographic separations on conventional HPLC systems with significant reductions in sample analysis time has been especially beneficial for laboratories tasked with the routine analysis of drug products. These laboratories typically have limited resources and can benefit greatly from faster separations and higher sample throughput. Ibuprofen is a weakly acidic, non-steroidal anti-inflammatory drug (NSAID) marketed under a variety of different trademark brands. Ibuprofen is available over the counter (OTC) in 200 mg doses in most countries (400 mg doses in some countries), and by prescription at higher doses. It is commonly used as an analgesic and anti-inflammatory, but can also relieve arthritis pain and reduce fever (antipyretic). At low doses (< 1200 mg/day), ibuprofen has the lowest incidence of digestive adverse drug reactions; however, common adverse effects include nausea, upset stomach or ingestion, and gastrointestinal ulceration or bleeding. Ibuprofen related compound C (4-isobutylacetophenone) is a known degradation product of ibuprofen that causes adverse effects in the central nervous system. As a result, its presence in ibuprofen tablets needs to be monitored and controlled over the shelf life of the product. The USP monograph for ibuprofen assay specifically requires the amount of ibuprofen related compound C in ibuprofen tablets to be limited to 0.1% per tablet.1 The ability to detect a low level concentration of such an impurity is critically impacted by the efficiency of the chromatographic column and we show here how to easily accomplish this with a Kinetex XB-C18 column based on the ultra-high efficiency core-shell particle technology.
Reagents and Chemicals
All reagents and solvents were HPLC or analytical grade. Chloroacetic acid, ammonium hydroxide and valerophenone were purchased from Sigma-Aldrich (Milwaukee, Wisconsin, USA). HPLC gGrade acetonitrile and water were purchased from Honeywell, Burdick & Jackson (Muskegon, Michigan, USA). USP ibuprofen reference standard (RS) and ibuprofen related compound C RS were purchased from US Pharmacopeia (Rockville, Maryland, USA).
Equipment and Materials
Columns Used: A fully porous 5 µm C18 250 × 4.6 mm column (as specified by the monograph) was compared with Kinetex 2.6 µm XB-C18 100 × 4.6 mm core-shell column.
Instrumentation: Agilent 1100 Series HPLC (Agilent Technologies Inc., Santa Clara, California, USA), equipped with quaternary gradient pump, autosampler, column oven and diode array detector (DAD).
Mobile Phase Preparation: 4.0 g of chloroacetic acid was dissolved in 400 mL of water (1.0 % w/v) and the pH adjusted to 3.0 with ammonium hydroxide, then 600 mL of acetonitrile was added and mixed well. This mobile phase mixture was filtered and degassed prior to use.
HPLC Conditions:
Column: Kinetex 2.6 µm XB-C18 100 Å
Dimensions: 100 × 4.6 mm and 75 × 4.6 mm
Part No.: 00D-4496-E0 and 00C-4496-E0
Mobile phase: Acetonitrile/Water (600:400) with 4 g Chloroacetic acid, pH 3.0
Flow-rate: 2.0 mL/min
Inj. Volume: 5 µL
Temperature: 30 °C
Detection: UV @ 254 nm
Sample: 1. Ibuprofen
2. Valerophenone (IS)
3. Ibuprofen related compound C
Standard Solution Preparation: A solution containing about 12 mg/mL of ibuprofen, 0.35 mg/mL of valerophenone (IS), and 0.012 mg/mL of ibuprofen related compound C was prepared by serial dilution in mobile phase.
Chromatographic Method: 1 µL of sample was injected with isocratic chromatographic separation using 60:40 acetonitrile/1.0 % (w/v) chloroacetic acid in water, pH 3.0 as the mobile phase at a flow-rate of 2.0 mL/min. The column was maintained at 30 ºC with UV detection at 254 nm.
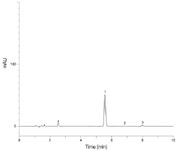
Figure 1: Ibuprofen assay on fully porous 5 µm C18 250 à 4.6 mm column, conditions as specified in monograph. Analytes: 1) Ibuprofen, 2) Valerophenone, 3) Ibuprofen related compound C.
Results and Discussion
The USP monograph for the assay of ibuprofen specifies using a 250 × 4.6 mm column packed with 5 µm media containing a C18 bonded phase and an isocratic mobile phase as outlined in the HPLC conditions above. The system suitability for this method requires resolution between ibuprofen and the internal standard (IS, valerophenone) and between IS and ibuprofen related compound C to be not less than (NLT) 2.5, and tailing factors for each peak to be not more than (NMT) 2.5. Figure 1 shows the chromatogram obtained running the USP method as specified. Resolution between ibuprofen and valerophenone, and between valerophenone and related compound C, is 7.73 and 5.93, respectively. Tailing factors (TF) are summarized in Table 1 and are well within the limit required to meet system suitability. At 2.0 mL/min the backpressure was 192 bar (2785 psi) and total analysis time was less than nine minutes.
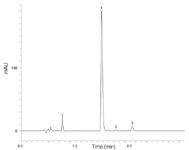
Figure 2: Ibuprofen assay on Kinetex 2.6 µm XB-C18 100 à 4.6 mm column, conditions as specified in monograph. Analytes: 1) Ibuprofen, 2) Valerophenone, 3) Ibuprofen related compound C.
The ultra-high efficiency expressed by Kinetex coreshell columns has been shown to be beneficial for several other pharmacopoeia methods2,3 in providing significant improvements in resolution. Figure 2 shows the chromatogram obtained on the 100 × 4.6 mm Kinetex 2.6 µm XB-C18 column using the same mobile phase and flowrate specified in the ibuprofen monograph. As expected, the shorter column length results in shorter retention times and a faster analysis time; however, there is no corresponding reduction in resolution. This is because the Kinetex core-shell column provides much higher efficiency and significantly narrow peaks, allowing for a shorter column to be used without sacrificing chromatographic resolution. Another benefit of the narrower peaks is the increase in signal intensity, easily allowing for the determination of low level impurities such as ibuprofen related compound C at the required limits.
Reducing the analysis time (from approximately nine to three minutes) with the Kinetex XB-C18 column while maintaining system suitability also provides significant benefits for the laboratory operation by reducing mobile phase solvent usage, while increasing productivity and throughput. Reducing solvent usage results in significant cost savings but also reduces solvent waste disposal costs.
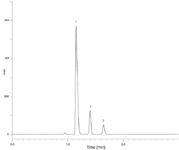
Figure 3(a): Ibuprofen assay on Kinetex 2.6 µm XB-C18 75 à 4.6 mm column @ 2.0 mL/min, conditions as specified in monograph. Analytes: 1) Ibuprofen, 2) Valerophenone, 3) Ibuprofen related compound C.
USP General Chapter <621> outlines the allowable adjustments that can be made to USP monograph methods.4 Here, the particle diameter has been reduced by the maximum allowed (50% smaller) and column length has been decreased by 60% (within the limit of 70%) to reduce analysis time and improve sensitivity, without sacrificing chromatographic resolution. Since pressure is directly proportional to column length, using a shorter column can significantly offset increases in pressure attributable to the smaller particle diameter.
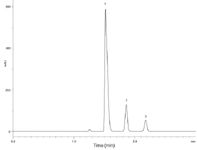
Figure 3(b): Ibuprofen assay on Kinetex 2.6 µm XB-C18 75 à 4.6 mm column @ 1.5 mL/min, conditions as specified in monograph. Analytes: 1) Ibuprofen, 2) Valerophenone, 3) Ibuprofen related compound C.
Figure 3(a) shows the chromatogram obtained using a 75 × 4.6 mm Kinetex 2.6 µm XB-C18 column. This column length is reduced by 70%, the maximum allowed per USP <621>, and the separation still meets the system suitability requirements (Table 1). Another adjustment that can reduce the backpressure is to reduce the flowrate, and USP <621> allows for a maximum adjustment to flowrate of ±50% (±1.0 mL/min in this case). Figure 3(b) shows the chromatogram obtained at a flow-rate of 1.5 mL/min (25% reduction in flowrate) with the 75 × 4.6 mm Kinetex column and the results are summarized in Table 1. Reducing the flowrate to 1.5 mL/min has the expected outcome of increasing analyte retention times; however, the overall analysis time is only increased to about 2.5 minutes, or less than onethird of the original monograph method runtime of nine minutes. Reducing the flow-rate reduces the backpressure to 228 bar, (~3455 psi). The analysis time is still significantly faster than the monograph method, so high productivity benefits can be realized in the laboratory.
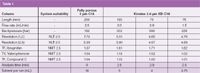
Table 1
Conclusion
For the assay of ibuprofen, the shorter 75 × 4.6 mm coreshell column and reduced flow-rate (1.5 mL/min) provide a good compromise for increasing throughput versus operating pressure. These benefits are achieved while maintaining system suitability for the assay of ibuprofen and fall within the allowable adjustments per USP <621>.
References
1. USP Monograph for Ibuprofen, USP33-NF28, 2010.
2. Phenomenex Technical Note TN-1071: Determination of Impurities and Related Substances for Acetylsalicylic Acid.
3. Phenomenex Technical Note TN-1090: Improved Throughput and Performance for the USP Assay for Propranolol Hydrochloride using Kinetex 2.6 µm C8 Core-Shell Column.
4. USP General Chapter <621>, USP33-NF28, 2010.

Phenomenex Inc.
411 Madrid Ave., Torrance, California 90501, USA
tel. +1 310 212 0555 fax +1 310 212 7768
Website: www.phenomenex.com

Separating Impurities from Oligonucleotides Using Supercritical Fluid Chromatography
February 21st 2025Supercritical fluid chromatography (SFC) has been optimized for the analysis of 5-, 10-, 15-, and 18-mer oligonucleotides (ONs) and evaluated for its effectiveness in separating impurities from ONs.



















