Rapid UHPLC Determination of Common Preservatives in Cosmetic Products
P-hydroxybenzoic acid esters, also called parabens, are widely used as antimicrobial preservatives in cosmetic products.
Njies Pedjie, PerkinElmer Inc.
P-hydroxybenzoic acid esters, also called parabens, are widely used as antimicrobial preservatives in cosmetic products. Parabens have a potential of endocrine effect — as a result, their use is limited in EU countries to a maximum concentration of 0.4% when used individually and 0.8% as a mixture. In the US, it is against the law to market products with ingredients that may cause injuries under label conditions, thus parabens are typically used at levels between 0.01 to 0.3%. This application note will present a fast, sensitive, and reliable UHPLC determination of six common parabens.
This application will discuss throughput improvements and reductions in solvent consumption compared to a conventional HPLC analysis of parabens. In addition, method conditions and performance data will be presented.
Experimental
The PerkinElmer Flexar FX-15 with Flexar UV-vis detector provided the UHPLC platform for this application. The separation was completed on a PerkinElmer Brownlee Analytical C18, 1.9 µm 50 mm x 2.1 mm column. The run time was approximately 3 min with a back pressure of 8100 psi (559 bar).
The separation was characterized and system calibrated with a mixture of parabens diluted from neat material. Repeatability was studied with six injections of the working standard (1200 ng/mL). Linearity was determined across the range of 30–1200 ng/mL. Samples of face hydrating lotion and cocoa butter body lotion were tested.
Results and Discussion
A conventional HPLC method would take approximately 16 min to resolve the parabens discussed here. This method would use approximately 24 mL of solvent.
Compared with conventional HPLC methodology, the UHPLC run time was dramatically shortened to about 3 min, the resolution of analyte peaks and sensitivity of the determination were improved and the total solvent usage was reduced to 2.1 mL per injection.
A representative chromatogram from standard solution analysis under UHPLC conditions is presented in Figure 1.
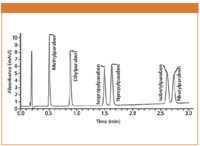
Figure 1: Example chromatogram from the analysis of a standard solution of common parabens with UHPLC and a 1.9 µm particle size column.
The method performance was outstanding. The linearity of the analysis achieved an average r2 value of 0.998. The average precision was less than 1% RSD, ranging from 0.9 to 0.2%.
Conclusion
The application of UHPLC to the analysis of common antimicrobials in cosmetics has resulted in a 13 min or 80% reduction in run time as well as a reduction of solvent usage of 22 mL, or about 90%. The PerkinElmer Flexar FX-15 UHPLC system and Brownlee Analytical C18, 1.9 µm 50 x 2.1 mm column resolved all the six parabens in about 3 min. The method was shown to be linear and precise. When applied to hand and body lotions, parabens were detected and their amounts complied with the EU and US Cosmetic recommendations.
References
(1) R. Golden, J. Gandy, and G. Vollmer, Critical Rev.Toxicol.35, 435–458 (2005).
(2) European Scientific Committee on Consumer Product (SCCP), SCCP/0873/05.
(3) Federal Food, Drug and Cosmetic Act (FD&C Act) Cosmetic Ingredient Review (CIR).

PerkinElmer Inc.
940 Winter Street, Waltham, MA 02451
tel. (800) 762-4000 or (203) 925-4602; fax (203) 944-4904
Website: www.perkinelmer.com

Infographic: Be confidently audit ready, at any time and reduce failures in pharma QC testing
November 20th 2024Discover how you can simplify the audit preparation process with data integrity dashboards that provide transparency to key actions, and seamlessly track long-term trends and patterns, helping to prevent system suitability failures before they occur with waters_connect Data Intelligence software.