Rapid Perfluorinated Alkyl Acid Analysis by LC–MS/MS Increases Sample Throughput
Special Issues
• Raptor C18 SPP 5 μm core-shell silica particle columns offer excellent resolution for fluorochemicals with short total cycle times. For even faster analysis, 2.7 μm core-shell particles are available. • Meets EPA Method 537 requirements. • Unique, robust Raptor C18 column design increases instrument uptime.
• Raptor C18 SPP 5 μm core-shell silica particle columns offer excellent resolution for fluorochemicals with short total cycle times. For even faster analysis, 2.7 μm core-shell particles are available.
• Meets EPA Method 537 requirements.
• Unique, robust Raptor C18 column design increases instrument uptime.
Perfluorinated alkyl acids are man-made fluorochemicals used as surface-active agents in the manufacture of a variety of products, such as firefighting foams, coating additives, textiles, and cleaning products. They have been detected in the environment globally and are used in very large quantities around the world. These fluorochemicals are extremely persistent and resistant to typical environmental degradation processes. As a result, they are widely distributed across the higher trophic levels and are found in soil, air, groundwater, municipal refuse, and landfill leachates. The toxicity, mobility, and bioaccumulation potential of perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA), in particular, pose potential adverse effects for the environment and human health.
Perfluorinated alkyl acid analysis can be challenging because these compounds are chemically different from most other environmental contaminants. They are difficult to quantify because some are more volatile than others, and they also tend to be more hydrophilic and somewhat reactive. In addition, fluorochemicals are present in polytetrafluoroethylene (PTFE) materials, so excluding the use of any PTFE labware throughout the sampling and analytical processes (including HPLC solvent inlet tubing) is essential for accurate analysis. Typically, perfluorinated alkyl acids are analyzed by LC–MS/MS methods, such as EPA Method 537, but long analysis times can significantly limit sample throughput.
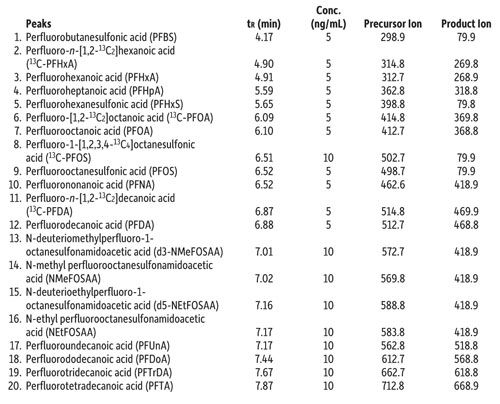
As written, the EPA 537 requires a 27-min cycle per sample, but the method does allow flexibility in the column used as long as there is sufficient resolution for the MS dwell time for all compounds in a specific retention time window. In Figure 1, all target perfluorinated alkyl acids were analyzed on a Raptor C18 column in under 8 min with a total cycle time of 10 min-resulting in an approximately three-fold faster analysis than the EPA method. While this analysis is significantly faster, there is no sacrifice in peak resolution or selectivity, meaning all fluorochemicals are easily identified and they elute as highly symmetrical peaks that can be accurately integrated and quantified by MS/MS. If PFOA and PFOS are the only target fluorochemicals, the analysis can be further optimized, which results in a fast, <2-min separation with a total cycle time of just 4.5 min, as shown in Figure 2.
Figure 1: Column: Raptor C18 (cat.# 9304512); Dimensions: 100 mm x 2.1 mm ID; Particle size: 5 μm; Pore size: 90 Å; Temp.: 40 °C; Sample: Diluent: Methanol–water (96:4); Conc.: 5–10 ng/mL; Inj. vol.: 5 μL; Mobile phase: A: 5 mM ammonium acetate in water; B: Methanol; Gradient (%B): 0.00 min (10%), 8.00 min (95%), 8.01 min (10%), 10.0 min (10%); Flow: 0.4 mL/min; Detector: MS/MS; Ion source: Electrospray; Ion mode: ESI-; Mode: MRM.
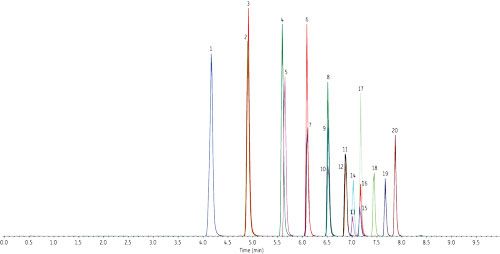
Figure 2: Column: Raptor C18 (cat.# 9304512); Dimensions: 100 mm x 2.1 mm ID; Particle size: 5 μm; Pore size: 90 Å; Temp.: 40 °C; Sample: Diluent: Water–methanol (50:50); Conc.: 5–10 ng/mL; Inj. vol.: 5 μL; Mobile phase: A: 5 mM ammonium acetate in water; B: Methanol; Gradient (%B): 0.00 min (60%), 2.50 min (95%), 2.51 min (60%), 4.50 min (60%); Flow: 0.4 mL/min; Detector: MS/MS; Ion mode: ESI-; Mode: MRM; Instrument: UHPLC.
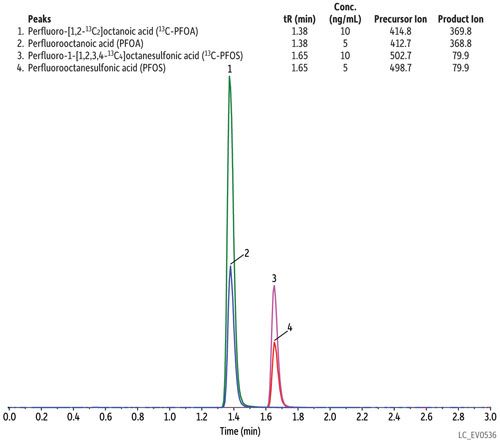
Table I: Peak identifications for Figure 1
Column description

Whether labs conducting perfluorinated alkyl acid analysis by LC use longer target analyte lists or focus just on PFOA and PFOS, the excellent peak shapes and separations achieved here result in consistent, accurate quantification with much shorter analysis times. By switching to a Raptor C18 column, labs can process more samples per hour while still meeting fluorochemical method requirements.

Restek Corporation
110 Benner Circle, Bellefonte, PA 16823
tel. (800) 356-1688, fax (814) 353-1309
Website: www.restek.com

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.
A Guide to (U)HPLC Column Selection for Protein Analysis
April 16th 2025Analytical scientists are faced with the task of finding the right column from an almost unmanageable range of products. This paper focuses on columns that enable protein analysis under native conditions through size exclusion, hydrophobic interaction, and ion exchange chromatography. It will highlight the different column characteristics—pore size, particle size, base matrices, column dimensions, ligands—and which questions will help decide which columns to use.












