Validation of Horizon Technology Disk Extraction Technology for US EPA Wastewater Method 625.1
Special Issues
The US EPA monitors a variety of chemicals in water that may cause harm to humans or wildlife to minimize exposure.
Zoe Grosser*, Alicia Cannon*, Michael Ebitson*, Melissa Lever*, Nic Rasnake†, Jessica Bowker†, Allen Fuller†, and Chris Johnson†, *Horizon Technology, and †ESC Lab Sciences
The US EPA monitors a variety of chemicals in water that may cause harm to humans or wildlife to minimize exposure. Method 625 was developed by the Office of Science and Technology in the Clean Water program to allow the monitoring of a large suite of semivolatile chemicals in wastewater for compliance with the National Pollution Discharge Elimination System (NPDES). NPDES is a system of permitting that defines the characteristics of water that is released into a waterway, defined by industrial category. The permitting levels are set depending on the waterway’s use. If the waterway is used for recreation or is an important wildlife habitat, the limit may be set lower.
The original method was developed in the early 1980s and has been updated several times since then to allow the use of more modern technology. The latest update has taken place over the last few years and was proposed in a Method Update Rule (MUR) in 2015 (1). The latest version of the method includes a larger suite of analytes (up to 364) and an extensive set of labeled surrogates to better monitor the method performance throughout the sample preparation and analysis step.
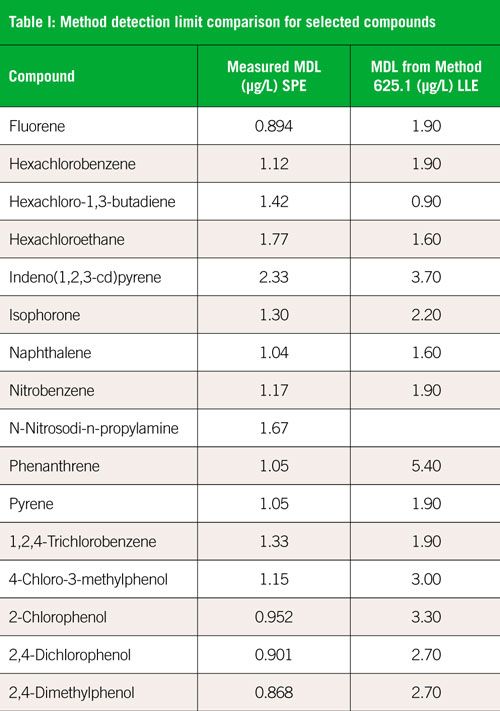
This application note will present the data collected as part of the demonstration of disk solid phase extraction validation for US EPA Method 625.1. Nine different wastewater matrices were evaluated and tested against the criteria listed in Table 6 of Method 625.1. Sample preparation was performed using the Atlantic® One-pass system, where the water sample is passed through a solid phase extraction (SPE) disk and carbon cartridge once, rather than twice with a pH change between loadings. Automation of the process was achieved using the SPE-DEX® 4790 system (superseded by SPE-DEX 5000). Table I shows method detection limits obtained in this work for selected compounds, compared with detection limits listed in the method. In most cases, the method detection limits are significantly better than those obtained using liquid-liquid extraction and older technology. Results for complex samples were also excellent and are detailed in the full application note (2).
References
- Method 625.1, December 14 revision, can be found in the MUR (February 19, 2015), or it can be downloaded here: https://nepis.epa.gov/Exe/ZyPDF.cgi/P100LVHC.PDF?Dockey=P100LVHC.PDF
- Application Note 117, “Validation of Horizon Technology Disk Extraction Technology for US EPA Wastewater Method 625.1,” available from www.horizontechinc.com.

Horizon Technology, Inc.
16 Northwestern Drive, Salem, NJ 03079
tel. (603) 893-3663
Website: www.horizontechinc.com

Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.












