A Rapid HPLC Method for Enabling PAT Application for Processing of GCSF
LCGC North America
The use of a monolithic column reduces analysis time in an on-line biopharmaceutical HPLC analysis of granulocyte colony stimulating factor.
This installment of "Biotechnology Today" focuses on developing a rapid method for reversed-phase high performance liquid chromatography (HPLC) analysis of granulocyte colony stimulating factor (GCSF) purity for process analytical technology (PAT) applications. It also highlights an approach that one could take to achieve faster HPLC analysis.
Progress in recombinant techniques, from many years of successful research, has led to the production of substantial amounts of novel protein- and peptide-based therapeutics. The evaluation of product quality (that is, identity, content, and purity) has a major role in the manufacturing process of biopharmaceuticals (1). The biotech production process itself usually shows high variability, which results in variability in product quality and, at times, lot failure. The heterogeneity that is typical in biotech therapeutics requires thorough characterization using multiple orthogonal techniques (2,3). This thorough characterization is a requirement for receiving regulatory approval to commercialize the product.
Of the many tools that are used, high performance liquid chromatography (HPLC) is the workhorse for analysis of biopharmaceutical proteins. The significant advantages that HPLC offers include high reproducibility, high sample throughput because of autosampling capabilities, high resolution of separation, and easy quantitation, high precision, and high robustness. Typical HPLC methods are 30–60 min long and are thus not amenable to high-throughput analysis. The focus of HPLC development in recent decades has been on reducing the time of analysis. Chromatographers have applied many different approaches to achieve this goal. Short columns with high flow velocities (4), high temperature (5–8), reduced particle size of packing (9), and ultrahigh pressure (10–13) are some of the approaches that have been used in both academic and industrial attempts to improve the speed of separation. The challenge is to reduce the separation time without significantly sacrificing the column efficiency or resolution. Simply shortening the column or increasing the mobile-phase velocity can result in shorter analysis time, but at the cost of reduced analyte retention time and lower efficiency.
Monolithic columns offer fast separations without compromising efficiency or resolution (14,15). The high permeability and large number of theoretical plates per unit pressure drop associated with monoliths are because of their most important and distinguishing physical features such as large through-pore size and skeleton ratios and high porosities (13). The short diffusion path length and high porosity supplied by the large through-pores not only lower the plate height, but also decrease the hydraulic resistance of the mobile-phase flow, thus reducing the pressure drop. The lower pressure drop permits operation at high flow rates on relatively long columns using a conventional HPLC system, but is not achievable with conventional packed columns. The efficiency of these monolithic columns to perform faster analysis is a necessary step in using HPLC as a process analytical technology (PAT) tool (16–19).
Lately, PAT has been receiving a lot of interest within the biopharmaceutical community because of the potential for continuous real-time quality assurance, resulting in improved operational control and compliance. It has been defined as a system for designing, analyzing, and controlling manufacturing through timely measurements (that is, during processing) of critical quality and performance attributes of raw and in-process materials and processes, with the goal of ensuring final product quality (20,21). The concept involves using on-line, at-line, or off-line analysis of critical quality attributes and process parameters to facilitate real-time decision making based on the measurement of critical quality attributes rather than surrogate measurements (22). The objective for PAT implementation could thus be one or more of the following (2,23):
- Better process understanding
- Improved yield because of prevention of the scrap, rejects, and reprocessing
- Reduction in the production cycle time by using on-line, at-line, or off-line measurements and control
- Decrease in the energy consumption and improved efficiency from conversion of the batch process into a continuous process
- Cost reduction because of reduced waste and reduced energy consumption
- Real-time release of the batches
A series of case studies has recently been published examining the use of on-line HPLC as a PAT tool, at pilot and manufacturing scales, for performing analyses to facilitate real-time decisions for column pooling based on product quality attributes (16). HPLC has also been proposed for the monitoring of protein purity in a refolding step to enable the timely ending of the step on the basis of product quality data (24). This column installment focuses on developing a rapid method for reversed-phase HPLC analysis of granulocyte colony stimulating factor (GCSF) purity for such PAT applications. It also highlights the approach that one could take to achieve faster HPLC analysis.
Materials and Methods
Protein and Reagents
GCSF, in the form of inclusion bodies, has been used in this study and has been obtained from a biosimilar producer. All reagents used were of HPLC grade or analytical grade. Trifluoroacetic acid, acetonitrile, methanol, and 2-propanol were purchased from Merck, Germany. For all analyses, MilliQ water (Millipore) filtered through a 0.22-µm membrane filter was used. Working standard and sample solutions of GCSF were prepared daily by diluting the reference standard and the sample of pharmaceutical formulation in solution A (0.1 M sodium acetate buffer, pH 4, containing 0.1 mg/mL of polysorbate, 80 and 50 mg/mL of sorbitol) to a final concentration of 0.2 mg/mL.
Chromatography System, Column, and Standard European Pharmacopoeia (EP) Method for GCSF
An Agilent 1200 Infinity Series HPLC unit was used consisting of a quaternary pump with degasser, an autosampler with a cooling unit, and a variable-wavelength detector. The experiments were carried out on a 100 mm × 4.6 mm Chromolith High Resolution RP-18 column (Merck) for method development and a 250 mm × 4.6 mm, 5-µm dp Phenomenex C4 column as a standard column of analysis. The HPLC sample injection volume was 20 µL and the detection of analytes was carried out at 215 nm using the variable-wavelength detector.
EP C4 Reversed-Phase HPLC Analysis Method: A 250 mm × 4.6 mm, 5-µm dp Phenomenex C4 column was equilibrated in 34:66 A–B mobile phase, with buffer A being 10% acetonitrile in Milli Q water with 0.1% trifluoroacetic acid and buffer B being 80% acetonitrile in Milli Q water, at a flow rate of 0.6 mL/min at 60 °C. Next, 50 µL of the filtered protein solution was injected onto the column, and the protein was eluted with a 66–90% linear gradient of mobile-phase B, in three column volumes. The elution profile was monitored at 215 nm.
Method Development
Method development included screening for organic solvent (acetonitrile, 2-propanol, methanol, or a combination thereof), pH (trifluoroacetic acid composition of 0.05% or 0.1%), flow rate (1, 2, and 3 mL/min), temperature (30 °C, 35 °C, and 40 °C), and gradient type (linear, concave).
Refolding Protocol
Refolding experiments were carried out by dilution. All operations were carried out at 25 °C. A sample of GCSF inclusion bodies was dissolved in a buffer containing urea and 0.05 M Tris, such that the optical density (OD) (280 nm) of the solubilized inclusion bodies after 10 times dilution in refolding buffer, would be in the range of 0.22–0.44 AU. This was followed by reduction using dithiothreitol for 30 min. The pH of the reduced inclusion bodies sample was then set to the desired value (8.2–10.2). Following reduction and pH adjustment, reduced inclusion bodies were diluted 10 times over a time period of 20 min in 0.05 M Tris buffer, containing 1.7 mM cystine. On dilution, initially the samples (50 µL each) were collected every 10 min for the first hour, followed by sample collection at every hour for the next 16 h. These samples were quenched with acetic acid to pH 4.0. Samples were then analyzed by HPLC using a monolithic column.
On-Line Sample Analysis During Refolding
A C18 Chromolith monolithic column (Merck Millipore International) was equilibrated with 77% methanol, 5% acetonitrile, and 18% water. A concave gradient was run from 77% methanol to 85% methanol for 4 min. Acetonitrile was kept at 5% throughout the gradient. At the end of the gradient, the percentage of methanol was increased to 100%, and finally re-equilibrated to the starting conditions of 77%, until the end of 5 min. Samples were taken during the refolding process and analyzed immediately. After the chromatogram indicated that GCSF was completely refolded, the refolding process was aborted.
Results and Discussion
The European Pharmacopoeia method is the standard method for reversed-phase HPLC analysis of GCSF. This is a high-resolution method and takes 60 min for the analysis of each sample at a flow rate of 0.6 mL/min. However, product quality–based, real time decision making requires rapid analysis. Hence, for our application, we targeted decreasing the analysis time for the reversed-phase HPLC assay using a monolithic column. In the following section, we examine the feasibility of using the reversed-phase HPLC technique with a monolithic column as an option for a PAT-based application.

Figure 1: Unresolved chromatogram using 2-propanol.
Developing a Rapid On-Line HPLC Method
As mentioned earlier, to achieve real-time decision making during protein refolding, it was necessary to reduce the analysis time of the reversed-phase HPLC assay. The off-line assay (EP method) had a turnaround time of 60 min and was operated at a flow rate of 0.6 mL/min. The capability of the monolithic column to allow use of higher flow rates was explored. To achieve resolution similar to the EP standard method, four solvents (2-propanol, methanol, acetonitrile, and water) and their combinations were tried. Results indicated that 2-propanol and its combination did not give the desired resolution (Figure 1). It was found that mobile phase containing methanol, acetonitrile, and water resulted in the desired resolution. Hence, a combination of acetonitrile (5% isocratic) and a gradient of methanol and water (77–85%) were chosen. The addition of 0.1% trifluoroacetic acid was found to further improve the resolution.
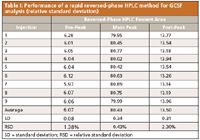
Table I: Performance of a rapid reversed-phase HPLC method for GCSF analysis (relative standard deviation)
The effect of increasing the flow rate on the monolithic column to 1, 1.5, and 3 mL/min was investigated. Multiple injections were performed to evaluate reproducibility of results. Table I displays the data obtained after analysis of the reference standard sample by rapid reversed-phase HPLC over multiple injections. It was seen that method variability, as measured by percent of relative standard deviation (%RSD) for this method was 0.43%. Even at a flow rate of 3 mL/min, adequate resolution was retained. For selection of the column temperature, the above method was run at 30 °C, 35 °C, and 40 °C. Resolution improved with increases in temperature. Because the maximum operational temperature for the monolithic column is 45 °C, 40 °C was chosen as the optimal temperature for this method. The linear gradient from 77% to 85% methanol was converted into a concave gradient, and this improved the resolution (Figure 2). A concave gradient helped in reducing the analysis time, without affecting resolution. The concave gradient was calculated as follows (25):

where θB is the volume fraction of stronger eluting solvent, t is the time after the gradient begins, tG is the total gradient time, and n is an integer controlling gradient steepness. For the concave gradient used in this method, the value of n was 3.5.
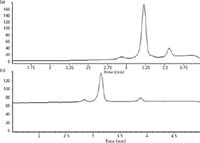
Figure 2: Comparison of (a) concave gradient and (b) linear gradient elution profiles to show better resolution and time reduction with the use of a concave gradient.
Elution was also tried in a flow-through mode for GCSF, but there was interference of the sample buffer. Flow-through elution is otherwise a very good option for time reduction. The main aim of this method was quantification of reduced and main GCSF during the refolding process, and this on-line HPLC method met the objective by rapidly analyzing every sample in 4 min.
Comparison Between the EP Reversed-Phase HPLC Method and the New Rapid HPLC Method
Figures 3a and 3b present a comparison of chromatograms of a representative reference standard that were generated via a standard EP method using a C4 HPLC column and a C18 monolithic column, respectively. The HPLC method using a C18 monolithic column was a very rapid (4 min) method, as compared to the EP method (60 min). In the EP method, acetonitrile was used for elution, which is an expensive solvent. The rapid HPLC method used methanol as the main eluent, thus resulting in considerable cost savings. The decrease in time of analysis also reduces energy and resource consumption.
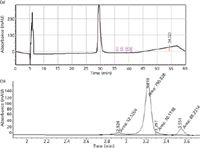
Figure 3: Chromatograms obtained using (a) the EP reversed-phase HPLC method with GCSF standard and (b) the rapid reversed-phase-HPLC method with GCSF standard.
Application of the Rapid HPLC Method as a PAT Tool in Refolding
At the beginning of the refolding process, the GCSF is in the reduced form and as the process advances it refolds into the native protein (26). In both the reversed-phase HPLC chromatograms, the peak following the main peak corresponds to the reduced form of GCSF (referred to as post-peak). Throughout the refolding process this reduced GCSF converted into native GCSF with time. Rapid HPLC was able to monitor the changes in the percent of purity at a much faster rate compared to the EP method, thus quickly indicating the completion of refolding. Figure 4 represents the gradual conversion of reduced GCSF into native GCSF with respect to time, demonstrating efficacy of the suggested scheme.

Figure 4: Application of PAT toward GCSF refolding.
Conclusions
The use of a monolithic column helps in reducing analysis times from 60 min to 4 min. This time savings enables the application of on-line HPLC in a PAT application, involving a protein refolding step. This time can be further reduced to a couple of minutes by using an ultrahigh-pressure liquid chromatography (UHPLC) system. The use of rapid HPLC will ensure that product quality is consistent from lot-to-lot as the process can be terminated, depending on the purity of the refolding pool. On-line HPLC also has applications in making real-time pooling decisions for process chromatography, as has been published earlier (16,17).
References
(1) O. Alexis, J.B. Farina, and M. Llabres. Curr. Pharm. Anal. 3, 230–248 (2007).
(2) A.S. Rathore, Trends Biotechnol. 27, 698–705 (2009).
(3) I.S. Krull and A.S. Rathore, LCGC North Am. 29(6), 454 (2010).
(4) U.D. Neue, J.L. Carmody, Y.F. Cheng, Z. Lu, C.H. Phoebe, and T.E. Wheat, Advances in Chromatography (Marcel Dekker, New York, 2001).
(5) F. Anitia, and Cs. Horvath, J. Chromatogr., A 435, 1–15 (1988).
(6) J.D. Thompson and P.W. Carr, Anal. Chem. 74, 4150–4159 (2002).
(7) B. Yan, J. Zhao, J.S. Brown, J.S. Blackwell, and P.W. Carr, Anal. Chem. 72, 1253–1262 (2000).
(8) N. Wu, Q. Tang, A.J. Lippert, and M.L. Lee, J. Microcolumn Sep. 13, 41–47 (2001).
(9) H. Chen and Cs. Horvath, J. Chromatogr., A 705, 3–20 (1995).
(10) J.E. MacNair, K.C. Lewis, and J.W. Jorgenson, Anal. Chem. 69, 983–989 (1997).
(11) N. Wu, D.C. Collins, J.A. Lippert, Y. Xiang, and M.L. Lee, J. Microcolumn Sep. 12, 463–469 (2000).
(12) N. Wu, J.A. Lippert, and M.L. Lee, J. Chromatogr., A 911, 1–12 (2001).
(13) Y. Xian, N. Wu, A.J. Lippert, and M.L. Lee, Chromatographia 55, 399–403 (2002).
(14) N. Wu, J. Dempsey, P.M. Yehl, A. Dovletoglou, D. Ellison, and J. Wyvratt, Anal. Chim. Acta 523, 149–156 (2004).
(15) Monolithic Silicas in Separation Science: Concepts, Syntheses, Characterization, Modeling and Applications, K.K. Unger, N. Tanaka, and E. Machtejevas, Eds. (Wiley-VCH, 2011).
(16) A.S. Rathore, M. Yu, S. Yeboah, and A. Sharma, Biotechnol. Bioeng. 100(2), 306–316 (2008).
(17) A.S. Rathore, R. Wood, A. Sharma, and S. Dermawan, Biotechnol. Bioeng. 101(6) 1366–1374 (2008).
(18) A.S. Rathore, A. Sharma, and D. Chilin. BioPharm Int. 19(8), 48–57 (2006).
(19) E.K. Read, J.T. Park, R.B. Shah, B.S. Riley, K.A. Brorson, and A.S. Rathore, Biotechnol. Bioeng. 105(2), 276–284 (2009).
(20) E.K. Read, J.T. Park, R.B. Shah, B.S. Riley, K.A. Brorson, and A.S. Rathore, Biotechnol. Bioeng. 105(2), 285-295 (2009).
(21) A.S. Rathore, R. Bhambure, and V. Ghare, Anal. Bioanal. Chem. 398, 137–154 (2010).
(22) U.S. Department of Health and Human Services, Food and Drug Administration "Guidance for Industry: PAT — A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance" (2004).
(23) B. Scott and A. Wilcock, J. Pharm. Sci. Technol. 60(1), 17–53 (2006).
(24) A.S. Rathore, A.S. Gerhardt, S.H. Montgomery, and S.M. Tyler, BioPharm Int. 22(1), 36–44 (2009).
(25) J.E. Haky, in Encyclopedia of Chromatography, Second Edition, J. Cazes, Ed. (CRC Press 2005), pp. 718–719.
(26) P. Bade, S.P. Kotu, and A.S. Rathore, J. Sep. Sci. 35, 3160–3169 (2012).
Varsha S. Joshi is a postdoctoral fellow at the Department of Chemical Engineering, at the Indian Institute of Technology Delhi.

Varsha S. Joshi
Vijesh Kumar is a graduate student at the Department of Chemical Engineering, at the Indian Institute of Technology Delhi.

Vijesh Kumar
Ira S. Krull is Professor Emeritus of Chemistry and Chemical Biology at Northeastern University, Boston, Massachusetts, and a member of LCGC's editorial advisory board.

Ira S. Krull
Anurag S. Rathore is a professor in the Department of Chemical Engineering at the Indian Institute of Technology in Delhi, India.

Anurag S. Rathore

Separating Impurities from Oligonucleotides Using Supercritical Fluid Chromatography
February 21st 2025Supercritical fluid chromatography (SFC) has been optimized for the analysis of 5-, 10-, 15-, and 18-mer oligonucleotides (ONs) and evaluated for its effectiveness in separating impurities from ONs.