Simultaneous Determination of Harpagoside and Its Metabolites in an Aspergillus niger Culture by Solid-Phase Extraction and HPLC
A new chromatography-based method for analyzing a traditional Chinese medicine and its metabolites.
When the traditional Chinese medicine harpagoside is cultivated with Aspergillus niger, it is transformed into its metabolite compounds harpagoside metabolite I, harpagoside metabolite II, and aucubinine B. This article describes a simple, rapid, and effective solid-phase extraction–high performance liquid chromatography method for the simultaneous determination of harpagoside and its three metabolites. The chromatographic separation was achieved on a C18 column by gradient elution. The mean absolute recoveries of the analytes were all over 99%. Quantification limits were 0.02 µg/mL for harpagoside, 0.01 µg/mL for harpagoside metabolite I, and 0.015 µg/mL for harpagoside metabolite II and aucubinine B. The method was then applied to quantifying the compounds during fermentation and evaluating the bioavailabilities of the three metabolites using a Caco-2 cell monolayer culture.
Harpagoside, a glycoside from Scrophularia ningpoensis Hemsl., Scrophularia modosa Linn, and Scrophularia buergeriana Miq (1,2), has been an important traditional drug in China for many years. Harpagoside has a wide range of therapeutic effects, such as relief from the pain induced by thermal stimulus (3), an anti-inflammatory effect (4), inhibition of TNF-α synthesis (5), antimicrobial activity against bacteria and yeasts (6), wound healing activity (7), and arthrosis pain relief (8). The structures of harpagoside, aucubinine B, harpagoside metabolite I, harpagoside metabolite II, and the related metabolic pathway (9,10) are shown in Figure 1.
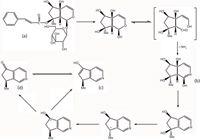
Figure 1: The structures of (a) harpagoside, (b) harpagoside metabolite I, (c) harpagoside metabolite II, (d) aucubinine B and the related metabolic pathway.
The determination of harpagoside by high performance liquid chromatography (HPLC) in carbon dioxide extracts of the secondary roots of Harpagophytum procumbens (11) and medicinal plants (12), and by HPLC–mass spectrometry (MS) in equine urine and plasma (13) have been described. But solid-phase extraction (SPE)–HPLC–UV for simultaneous determination of harpagoside and its metabolites in a broth of microorganisms has not been reported. This article describes a method based on SPE–HPLC–UV for simultaneous determination of harpagoside and its metabolites harpagoside metabolite I, harpagoside metabolite II, and aucubinine B in an Aspergillus niger culture. The method was then successfully applied to the determination of the time course of the biotransformation process and evaluation of the bioavailability of harpagoside and its metabolites.
Experimental
Materials
Harpagoside, harpagoside metabolite I, harpagoside metabolite II, and aucubinine B standards were supplied by the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). All of the standards had a purity of >99%. HPLC-grade acetonitrile and methanol were purchased from Concord Tech. (Tianjin, China). Analytical-grade acetic acid was obtained from Tianjin Chemical Reagent Co. (Tianjin, China). The water was deionized using a Type 2 Millipore water purification system.
Culture Medium
Aspergillus niger was isolated and purified from rotted apples, maintained in potato dextrose agar slants, and subcultured every two months. The compositions of the medium for biotransformation and method development was as follows: 20 g/L glucose, 3 g/L peptone, 2.5 g/L yeast extract, 4 g/L monobasic potassium phosphate, 1 g/L sodium chloride, 1 g/L magnesium sulfate heptahydrate, and 1.5 g/L manganous sulfate, all adjusted to pH 7.0.
Instruments and Chromatographic Conditions
The chromatographic system consisted of a binary HPLC pump (Waters), a model 2996 photodiode-array detector (Waters), and a manual injector. A 200 mm × 4.6 mm stainless steel column was packed with Nucleosil C18 (Machery-Nagel) stationary phase with a particle size of 5 µm. The column was maintained at 30 °C and the UV detection wavelength was 278 nm. Mobile-phase A was 0.2% acetic acid in acetonitrile (v/v), and mobile-phase B was 0.2% acetic acid in water (v/v). The gradient was as follows: 0–8 min, 3–65% A; 8–14 min, 65–92% A; 14–18 min, 92% A; and 20–30 min, 90–3% A. The samples were filtered through 0.45-µm membrane filters. The injection volume was 10 µL, and the mobile-phase flow rate was 1 mL/min.
Standard Preparation
Individual stock solutions of harpagoside, harpagoside metabolite I, harpagoside metabolite I, and aucubinine B were prepared in methanol and stored in closed volumetric flasks at 4 °C. No change in stability over a period of 36 h was observed. The working solutions were prepared by diluting appropriate portions of these four solutions with methanol at concentrations of 2.5, 1.0, 0.5, 0.1, 0.05, 0.04, 0.02, 0.015, and 0.01 µg/mL.
Sample Preparation
The calibration curves of harpagoside, harpagoside metabolite I, harpagoside metabolite II, and aucubinine B were constructed independently. The standard solutions were prepared with 2 mL of methanol at concentrations of 2.5, 1.0, 0.5, 0.1, 0.05, 0.04, 0.02, 0.015, and 0.01 µg/mL. All samples (each 2 mL) were dried with nitrogen and mixed with cultures of A. niger that had been cultivated for 48 h. The mixtures were vortex-mixed for 2 min and centrifuged at 20,000 rpm for 20 min, and the supernatant was cleaned with Oasis HLB SPE cartridges (30 mg, 1 mL, Waters). The cleanup procedure was as follows: 0.5 mL of supernatant was diluted with 1 mL of 1 M phosphate buffer (pH 7.0). After a brief vortex mixing step at room temperature and centrifugation at 20,000 rpm for 20 min, the resulting supernatant was applied to an SPE cartridge previously activated with 1 mL of methanol and conditioned with 1 mL of deionized water. The cartridge was washed with 1 mL of 5% methanol and then with 1 mL of 100% methanol. The eluate was dried with nitrogen and reconstituted in 100 µL of 3% acetonitrile in water (v/v), and 10 µL was injected for HPLC analysis.
Calibration Curves
The calibration range for each of the four compounds was 0.01–2.5 µg/mL. Calibration curves were obtained by plotting the peak areas versus concentrations. The regression equations were calculated by the least-squares method.
Results and Discussion
Method Development
The effects of different concentrations of formic acid, acetic acid, and phosphoric acid on the chromatography behavior were studied and compared. The results showed that 0.2% acetic acid and 1% phosphoric acid provided satisfactory chromatography. Acetic acid was finally chosen because 1% phosphoric acid gave a pH below 2 and would damage the chromatographic column. Under the chromatographic conditions described in this article, the peaks of harpagoside, harpagoside metabolite I, harpagoside metabolite II, and aucubinine B were well resolved. Their retention times were 7.2, 10.7, 12.9, and 15.4 min, respectively. The components of blank medium did not yield any interfering peaks.
Analyte Stability
To determine the stability of harpagoside and its three metabolites in methanol under the analysis conditions, the solutions of harpagoside (0.5 µg/mL), harpagoside metabolite I, harpagoside metabolite II, and aucubinine B (0.1 µg/mL) were incubated at 30 °C and taken out for assay every 2 h during a 36-h period. The results showed that all of these four compounds are stable in methanol under experimental conditions.
Linearity
Linearity was tested at nine concentration points ranging from 0.01 to 2.5 µg/mL for all of the analytes. Respective regression equations were as follows: y = 52.32x – 89.76 for harpagoside, y = 32.52x – 45.26 for harpagoside metabolite I, y = 14.73x – 25.64 for harpagoside metabolite II, and y = 41.253x – 33.71 for aucubinine B. The correlation coefficients for the analyte calibration data were 0.9999, 0.9999, 0.9998, and 0.9999, respectively.
Limit of Quantification
The limit of quantification was defined as the lowest amount detectable with a precision of less than 10% (n = 6) and an accuracy of ±10% (n = 6). Thus the limit of quantification was found to be 0.02 µg/mL for harpagoside — which was lower than reported in other studies (14) — 0.01 µg/mL for harpagoside metabolite I, and 0.015 µg/mL for harpagoside metabolite II and aucubinine B in the medium.
Recovery
The absolute recoveries of harpagoside, harpagoside metabolite I, harpagoside metabolite II, and aucubinine B were evaluated by comparing the areas determined in the medium spiked with known amounts of the analytes, which had been processed through the entire cleanup procedure described above, to the areas from the standard solutions prepared in methanol. The absolute recoveries were 103.0%, 99.7%, and 99.9% at concentrations of 0.01, 0.1, and 2.5 µg/mL for harpagoside; 102.1%, 99.8%, and 99.9% at 0.01, 0.5, and 2.5 µg/mL for harpagoside metabolite I; 99.3%, 99.6%, and 99.6% at 0.015, 0.5, and 2.5 µg/mL for harpagoside metabolite II; and 100.6%, 100.7%, and 99.9% at 0.015, 0.5, and 2.5 µg/mL for aucubinine B.
Precision and Accuracy
The precision and accuracy were assessed by analyzing samples at three concentrations (low, middle, and high levels). The precision was based on the calculation of the relative standard deviation (RSD). An indication of accuracy was based on the calculation of the relative error of the mean of found concentration as compared with the nominal concentration. The intraday and interday precision and accuracy of harpagoside and its metabolites are shown in Table I. The RSD for all samples analyzed was within 7.0%, and the relative error ranged from 1.3% to 4.7% of the nominal concentrations.
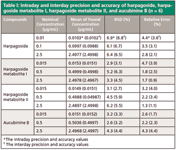
Table I: Intraday and interday precision and accuracy of harpagoside, harpagoside metabolite I, harpagoside metabolite II, and aucubinine B (n = 6)
Determination of Analyte Concentrations in the A. niger Culture
Harpagoside dissolved in methanol was added into a culture of A. niger that had been cultivated for 2 days. The final concentration of harpagoside in the culture was 0.5 mg/mL. The samples were processed as described earlier and were analyzed by the proposed method. The samples that were cleaned with the SPE cartridges were compared with those not processed with the cleanup procedure, and the result is shown in Figure 2. In the chromatogram of the sample not cleaned by the SPE procedure (Figure 2b), the resolution for harpagoside and its metabolites is not satisfactory and the culture components yielded some interfering peaks. The chromatogram shown in Figure 2a suggests that components in the culture interfering with the target compounds were removed by the SPE step, and harpagoside and its metabolites are well resolved. Thus we concluded that the cleanup procedure using SPE was critical to the proposed analysis method. The time course of the biotransformation is shown in Table II. The results indicated that harpagoside was rapidly transformed into the three metabolites during the 5 days of fermentation.
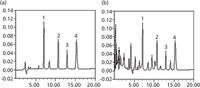
Figure 2: HPLC separations of harpagoside and metabolites: (a) The sample collected at 120 h and cleaned with SPE; (b) the sample collected at 120 h but not processed by the SPE cleanup procedure. Peaks: 1 = harpagoside, 2 = harpagoside metabolite I, 3 = harpagoside metabolite II, 4 = aucubinine B.
Bioavailability Evaluation
A Caco-2 monolayer maintained in completed Dulbecco's modified Eagle's medium (15) for 21 days with a transepithelial electrical resistance greater than 400 O/cm2 was used for the transport experiment. The stock solution of a mixture of harpagoside, harpagoside metabolite I, harpagoside metabolite II, and aucubinine B was prepared by dissolving them (15 mg each) in 50 mL of Hanks' balanced salt solution (16), and the concentration was 300 µg/mL for all of them. A 1.5-mL volume of stock solution was added into the apical compartment, and 0.6 mL of sample was taken from the basolateral compartment after 0, 30, 60, 90, 120, and 150 min. The samples were cleaned with cartridges and were analyzed by the proposed method. Typical chromatograms are shown in Figure 3. The calibration equation was y = 48.32x – 82.66 for harpagoside, y = 36.37x – 39.87 for harpagoside metabolite I, y = 16.91x – 22.34 for harpagoside metabolite II, and y = 39.27x – 28.99 for aucubinine B. The concentrations of harpagoside and its metabolites are shown in Table III. The results showed that the apparent permeability coefficient of harpagoside metabolite II is the largest, which means that harpagoside metabolite II has a higher bioavailability than harpagoside and the other metabolites.
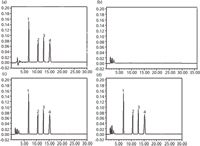
Figure 3: Chromatography of harpagoside and its metabolites: (a) standard spiked with harpagoside, harpagoside metabolite I, harpagoside metabolite II, and aucubinine B dissolved in Hank’s balanced salt solution; (b) blank Dulbecco’s modified Eagle’s medium; (c) blank Dulbecco’s modified Eagle’s medium spiked with harpagoside (0.5 µg/mL), harpagoside metabolite I (0.3 µg/mL), harpagoside metabolite II (0.3 µg/mL), and aucubinine B (0.3 µg/mL); (d) samples taken from the basolateral compartment at 90 min. Peaks: 1 = harpagoside, 2 = harpagoside metabolite I, 3 = harpagoside metabolite II, 4 = aucubinine B.
Conclusions
Harpagoside has been an important Chinese traditional drug for many years. Most studies of harpagoside have focused on the isolation and determination of the compound from herbs and the pharmacology of harpagoside. In our investigation of harpagoside biotransformation, the compound was found to be metabolized into three metabolites when cultured with A. niger. This article describes the first simple and effective SPE–HPLC–UV method for simultaneous determination of harpagoside and its three metabolites. The intraday and interday precision and accuracy of these compounds showed that the RSD for all samples analyzed was within 7.0% and the relative error ranged from 1.3% to 4.7%. The retention time for harpagoside using our method was much shorter than that reported in other studies (17,18). The precision and accuracy for harpagoside were lower than values obtained in studies in which harpagoside and cinnamic acid were determined by ultrahigh-pressure liquid chromatography (UHPLC)–MS-MS (19) but higher than those obtained using an HPLC method (20). However, the HPLC–UV method described in this article is simpler and faster and thus is more reproducible. The method for the analysis of harpagoside and its metabolites in cultures of A. niger and Caco-2 was specific, sensitive, and accurate. It was successfully applied to the bioavailability evaluation of harpagoside and its metabolites using the Caco-2 cell monolayer model.
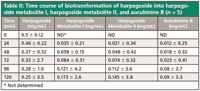
Table II: Time course of biotransformation of harpagoside into harpagoside metabolite I, harpagoside metabolite II, and aucubinine B (n = 5)
Acknowledgments
We thank the fund of GJJ10593 and 10JCZDJC21400 for financial support.

Table III: Time course of transport of harpagoside, harpagoside metabolite I, harpagoside metabolite II, and aucubinine B (n = 5)
References
(1) X.W. Yang and M.Y. Hao, Metabolite Analysis for Chemical Constituents of Traditional Chinese Medicines (China Medical Science Press, Beijing, 2003), pp. 289–303.
(2) S.Q. Tong, J.Z. Yan, and J.Z. Lou, Phytochem. Anal. 17, 406–408 (2006).
(3) M.L. Andersen, E.H. Santos, M.L. Seabra, A.A. deSilva, and S. Tufik, J. Ethnopharmacol. 91, 325–330 (2004).
(4) M. Gunther, S. Laufer, and P.C. Schmidt, Phytochem. Anal. 17, 1–7 (2006).
(5) B.L. Fiebich, M. Heinrich, K.O. Hiller, and N. Kammerer, Phytomedicine 8, 28–30 (2001).
(6) S. Weckesser, K. Engel, B. Simon-Haarhaus, A. Wittmer, K. Pelz, and C.M. Schempp, Phytomedicine 14, 508–516 (2007).
(7) C.S. Philip, S.J.S. Monique, S. Julia, J.H. Peter, and G. Peter, Phytotherapy Research 16, 33–35 (2002).
(8) T. Wegener and N.P. Lüpke, Phytotherapy Research 17, 1165–1172 (2003).
(9) X.W. Yang, C.T. Zou, and M. Hattori, Chinese Chemical Letters 11, 779–782 (2000).
(10) X.W. Yang and M.Y. Hao, Metabolite Analysis for Chemical Constituents of Traditional Chinese Medicines, T.K. Huang, Ed. (China Medical Science Press, Beijing, 2003), pp. 289–303.
(11) M. Gunther and P.C. Schmidt, J. Pharm. Biomed. Anal. 37, 817–821 (2005).
(12) H.S. Alexander, J. Chromatogr. A 1073, 377–381 (2005).
(13) C. Colas, P. Garcia, M.A. Popot, Y. Bonnaire, and B. Stephane, Rapid Commun. Mass Spectrom. 20, 3257–3266 (2006).
(14) Y. Li, C. Zou, and H. Liu, Chromatographia 50, 358–362 (1999).
(15) F.J. Li, H. Li, C.I. Mau, R. Chan, H. Than, C. Dovrak, and C.J. Yee, J. Pharma. Sci. 95, 1318–1325 (2006).
(16) C. Hilgendorf, H. Spahn-Langguth, and C.G. Regardh, J. Pharma. Sci. 89, 63–75 (2000).
(17) A.H. Schmidt, J. Chromatogr. A 1073, 377–381 (2005).
(18) M.K. Lee, O.G. Choi, J.H. Park, H.J. Cho, M.J. Ahn, S.H. Kim, Y.C. Kim, and S.H. Sung, J. Sep. Sci. 30, 2345–2350 (2007).
(19) Z. Xiong, Y. Fu, J. Li, F. Qin, and F. Li, Chromatographia 72, 163–169 (2010).
(20) P. Li, Y. Zhang, L. Xiao, X. Jin, and K. Yang, Anal. Bioanal. Chem. 389, 2259–2264 (2007).
Jun Chang is with the School of Life Science at the Jiangxi Science & Technology Normal University in Nanchang, People's Republic of China.
Zhou Bin is with the School of Pharmacy at the Jiangxi Science & Technology Normal University in Nanchang, People's Republic of China.
Direct correspondence to: changjun8772@163.com

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)