QuEChERS - A New Sample Preparation Technique for Multiresidue Analysis of Pesticides in Foods and Agricultural Samples
LCGC North America
This month's installment of "Sample Prep Perspectives" describes a new extraction technique called QuEChERS (standing for quick, easy, cheap, effective, and safe and is pronounced "catchers") for the sample preparation of pesticides in foods and agricultural samples.
Pesticide residue analysis of food and environmental samples has been performed for over 40 years. Most pesticide methods are not oriented toward measuring a single pesticide. Instead, residue-monitoring laboratories are geared to perform multiclass, multiresidue methods to detect a wide variety (in the hundreds) of pesticides potentially in the sample. Because of the wide range of chemical properties of pesticides (including acidic, basic, and neutral) and the wide variety of matrices (polar, nonpolar, fatty, waxy, and so forth), the sample initially must be cleaned up using a compatible sample preparation technique before injection into the chromatographic system. Ideally, a multiresidue method should be fast and easy to perform, require a minimum amount of chemicals (especially solvents), provide a certain degree of selectivity, and still cover this wide array of analyte–matrix pairs. Although many sample preparation protocols involve lengthy multistep procedures, if the number of steps can be minimized by use of a simple sample preparation procedure, reproducibility (precision) and accuracy can be improved and time can be saved.

Ronald E. Majors
For many years, the standard approach for pesticides multiresidue methods for foods and agricultural products involved the use of organic solvent extraction (usually acetone), followed by water dilution and partitioning into a nonpolar solvent (such as methylene chloride and petroleum ether). This approach worked fine for nonpolar pesticides but certain polar compounds such as organophosphorus insecticides and several modern pesticides were partially lost. More sophisticated approaches were needed and analysts began to experiment with different organic solvents for the initial extractions. Next, the addition of various salts to the mixtures was found to affect recovery by changing solvent polarity and this became the fashionable approach (salting out effect). Some of these methods became the standard official multiresidue methods used in government, contract, and testing labs today.
With environmental and health concerns about the use of chlorinated solvents, workers investigated various non-halogen-containing solvents and solvent mixtures such as ethyl acetate, acetonitrile, and cyclohexane–ethyl acetate for extraction. For various reasons, none of these procedures gave an entirely satisfactory set of results, particularly in the area of extraction efficiency for wide ranges of pesticides. Extraction solvent compatibility with the analytical technique, be it high performance liquid chromatography (HPLC) or gas chromatography (GC) with and without mass spectrometry (MS) detection was another complicating problem.
Therefore, new extraction techniques were devised for solid samples, including supercritical fluid extraction, microwave-assisted extraction, solid-phase microextraction, matrix solid-phase dispersion, and pressurized fluid extraction–accelerated solvent extraction. Although most of these techniques used less organic solvent than conventional extraction, some were slow and most of the instrumental techniques were run in a serial manner, were of high cost, and required specialists to develop and troubleshoot methods. Some of these extraction techniques lacked sufficient selectivity (microwave-assisted and pressurized fluid extraction), and sample sizes were limited, an important consideration for trace analysis. Supercritical fluid extraction was particularly problematic with each analyte–matrix requiring a different set of experimental conditions and the technique was susceptible to varying water content among the samples. Some of these methods involved considerable cleanup of glassware and extraction vessels before the next use.
The need for a simple, rapid, inexpensive, multiclass multiresidue method that provided high quality results with a minimal number of steps, with reduced reagent use, and required little glassware led Anastassiades and coworkers (1) to develop a new method for the sample preparation of pesticide residues in fruits and vegetables. They gave the method the name QuEChERS, which stands for quick, easy, cheap, effective, and safe. The technique has attracted the attention of pesticide laboratories worldwide. Official methods from AOAC International and the Committee of European Normalization (CEN) are now available. Although one easily can assemble the necessary materials from a general laboratory catalog to perform QuEChERS — convenient kits have now been assembled commercially by Sigma Aldrich/Supelco (Bellefonte, Pennsylvania), Restek (Bellefonte, Pennsylvania), and United Chemical Technologies (Bristol, Pennsylvania).
The purpose of this article is to give an introduction to QuEChERS by providing some background information, typical samples and types of pesticides handled, quantitative possibilities, and applications examples. We will contrast the technique to matrix solid-phase dispersion, which has some similarities.
What is QuEChERS?
QuEChERS is a sample preparation approach entailing solvent extraction of high-moisture samples with acetonitrile, ethyl acetate, or acetone and partitioning with magnesium sulfate alone or in combination with other salts followed by cleanup using dispersive-SPE. It is very flexible and since its inception (1), there have been several modifications of the technique depending on analytes, matrices, instrumentation, and analyst preferences. I shall explain through a flow diagram and pictures how this simple procedure is performed. Basically, the sample is first extracted with a water-miscible solvent (for example, acetonitrile) in the presence of high amounts of salts (for example, sodium chloride and magnesium sulfate) and buffering agents (for example, citrate) to induce liquid separation and stabilize acidic and basic labile pesticides, respectively. Upon shaking and centrifugation, an aliquot of the organic phase is subjected to further cleanup using dispersive SPE (adding small amounts of bulk SPE packing sorbents to the extract). After sample cleanup, the mixture is centrifuged and the resulting supernatant can be analyzed directly or can be subjected to a concentration and solvent exchange step if necessary.
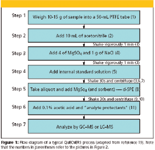
Figure 1
Referring to Figures 1 and 2, which outline the process more clearly in a flow diagram and pictorially, one can understand the simplicity of the technique. However, as with any sample preparation technique, one still must optimize the method so some method development might be required. Compromises have to be made to ensure method simplicity, speed, broad applicability, high recovery, and adequate selectivity. Anastassiades and coworkers (1) have studied the factors affecting these analytical requirements, and they are summarized in the following:
- Sample type
- Extraction solvent(s)
- Sample:solvent ratio
- Type(s) of salt added
- pH
- Comminution
- Water content
- Shaking versus blending
- Amt. of salt(s) added
- Time of extraction
Step 1: Sample Comminution
Because the sample mass (10–15 g) used in the QuEChERS technique is reduced compared with more traditional extraction approaches, it is of utmost importance to ensure that the original sample, that is typically kilograms, is extremely homogeneous. Thus, a powerful chopping device is recommended to homogenize the sample to maximize surface area and to ensure better extraction efficiencies. Such a homogenization procedure will ensure that the 10–15-g subsample is representative of the original. To prevent loss of the more volatile pesticides, the use of dry ice during the homogenization step is highly recommended.

Figure 2
Fruit and vegetable samples contain between 80–95% water and steps 2 and 3 will ensure that a phase separation between this water and an organic solvent takes place so that the pesticides of interest will be in the organic phase.
Step 2: Extraction–Partitioning
Although other nonhalogenated solvents such as acetone and ethyl acetate may be used, acetonitrile is the recommended solvent for QuEChERS because, upon the addition of salts, it is separated more easily from water than acetone. Ethyl acetate has the advantage of partial miscibility with water but it co-extracts lipids and waxes, obtains lower recoveries for acid–base pesticides, and provides less cleanup in dispersive-SPE. Acetonitrile extracts less of the lipophilic materials. However, samples with high sugar content, acetonitrile, and water can form two phases (1). Compared with acetone, the use of acetonitrile allows the better removal of residual water with magnesium sulfate. It is compatible with HPLC mobile phases and GC applications, although it tends to give a large solvent expansion volume during GC vaporization, interferes with nitrogen-specific GC detectors, and is less volatile than the other common organic solvents, thus making evaporative concentration steps more time consuming.
Step 3: Addition of Salts
The purpose of salt addition is to induce phase separation. The salting-out effect also influences analyte partition, which is dependent upon the solvent used for extraction (Step 3). The concentration of salt can influence the percentage of water in the organic phase and can adjust its "polarity." In QuEChERS, acetonitrile alone often is sufficient to perform excellent extraction efficiency without the need to add nonpolar cosolvents that dilute the extract and make the extracts too nonpolar. By using deuterated solvents and nuclear magnetic resonance, Anastassiades and colleagues (1) investigated the effect of various salt additions on recovery and other extraction parameters. They studied the effect of polarity differences between the two immiscible layers. The use of magnesium sulfate as a drying salt to reduce the water phase helped to improve recoveries by promoting partitioning of the pesticides into the organic layer. To bind a significant fraction of water, the amount of magnesium sulfate exceeded saturation concentration. The supplemental use of sodium chloride helps to control the polarity of the extraction solvents and thus influences the degree of matrix cleanup of the QuEChERS method but too much of this salt will less the organic layer's ability to partition polar pesticides.
In some instances, the pH of the extraction must be controlled. Most, but not all, pesticides are more stable at lower pH. For certain problematic pesticides, such as those that are strongly protonated at low pH, the extraction system must be buffered in the range of pH 2–7 for successful extractions (4). Of course, the pH at which the extraction is performed also can influence the coextraction of matrix compounds and pesticide stability.
Step 4: Internal Standard Addition
To minimize error generation in the multiple steps of the QuEChERS method, an internal standard is often added to the process. For most of the development work, the original authors (1) used tri-phenylphosphate, which had the right properties to undergo quantitative extraction for low fat matrices. A more complete study of various internal standards was undertaken by Anastassiades (3), who recommended the use of more than one internal standard as quality control measures to enable recognition of errors due to mispipetting or discrimination during partitioning or cleanup. In most cases, the internal standard is employed at an early stage of the analytical procedure. However, in the case of samples with high fat content, the excessive fat can form an additional layer into which analytes can partition. In the presence of elevated fat amounts (for example, > 0.3 g of fat/10 mL of acetonitrile), it was recommended to employ the internal standard at the end of the procedure (assuming the volume of the organic phase is exactly 10 mL).
Step 5: Dispersive solid-phase extraction
Traditionally, SPE cleanup used plastic cartridges containing various amount of sorbent material. In dispersive solid-phase extraction, an aliquot of sample extract (for example, 1 mL) is added to a vial containing a small amount of SPE sorbent (50 mg of primary secondary amine, PSA) and the mixture is shaken or mixed on a vortex mixer to evenly distribute the SPE material and facilitate the cleanup process. The sorbent is then separated by centrifugation and an aliquot of the supernatant is subjected to analysis. The sorbent is chosen to retain matrix components and not the analytes of interest. In some cases, other sorbents or mixed sorbents can be used. For samples with high fat, PSA mixed with a C18 sorbent is recommended while for samples with moderate and high levels of chlorophyll and carotinoids (for example, carrots, romaine lettuce), PSA mixed with graphitized carbon black at various ratios of sorbents is used. Although the addition of graphitized carbon black helps with the partial removal of chlorophyll, there is an accompanying partial loss of certain structurally planar analytes, so this process in a balancing act.
Dispersive solid-phase extraction is similar in some respects to matrix solid-phase dispersion developed by Barker (4–6), but in this case, the sorbent is added to an aliquot of the extract rather than to the original solid sample as in matrix solid-phase extraction. In dispersive solid-phase extraction, a smaller amount of sorbent is used because only an aliquot of the sample is subjected to the cleanup. Compared with SPE, dispersive solid-phase extraction takes less time and uses less labor and lower amounts of solvent. One need not worry about channeling, analyte or matrix breakthrough, or preconditioning of SPE cartridges. Just as a drying agent is sometimes added to the top of an SPE cartridge, magnesium sulfate is added simultaneously with the SPE sorbent to remove much of the excess water and improve analyte partitioning to provide better cleanup.
Step 6: Add Acetic Acid and "Analyte Protectants"
This optional step is found to be most useful for pesticides that are unstable at intermediate pH values and for analytes that might tail or breakdown on the capillary GC column interior surfaces, on sorbed nonvolatile compounds from previous injection, on the inlet liner or on the precolumn (guard column). In this case, analyte protectants are added to the extracts before GC. The protectant compounds are chosen so that they do not interfere with the separation of the pesticides of interest yet will cut down on interactions of these pesticides with active groups in the GC flowsteam. Thorough studies were devoted to selecting the appropriate analyte protectants (7-8), and a combination of sorbitol, gulonolactone, and ethylglycerol were found to cover the entire range of pesticides. The hydroxyl groups of these protectants interacted with active sites on the chromatographic column and in the flowstream and enhanced the pesticide analyte response. The results demonstrated that errors in GC analysis caused by matrix effects also were reduced dramatically with the help of analyte protectants. Of course, with LC and LC–MS, the protectants are not be required.
Step 7: Analysis
Often, the sample aliquot from Step 6 can be injected directly into a GC or HPLC system without further workup. For example, for LC–MS analysis, it might be necessary to add formic acid to provide better MS sensitivity or for GC–MS analysis, and if the instrument is not equipped with a programmable temperature vaporizer, evaporation of the supernatant with reconstitution in toluene might be needed.

Table I: Extraction procedure used in example-pesticides in oranges (9)
Application of QuEChERS — Pesticides from Oranges
To illustrate a typical application of QuEChERS, a commercially available kit was used to quantitatively extract 29 pesticides from oranges (9). Oranges for extraction were obtained from a local grocery store and were not labeled as "organic." Four extracts were prepared from orange skins according to the procedure summarized in Table I. An extract spiked only with internal standard (ethoprophos) at 100 ng/g served as a control. Three replicate extracts were spiked with each pesticide plus the internal standard (each at 100 ng/g) and used to determine the accuracy and precision of the method. The final extracts were solvent exchanged from acetonitrile to toluene to increase the sensitivity of the GC–MS analysis. Vials containing preweighed salts and sorbent (Sigma Aldrich/Supelco) were used to perform the extraction and cleanup procedures. The GC–MS conditions used for analysis are shown in Figure 3.

Figure 3
The chromatograms for the spiked orange samples are presented in Figure 3. Several background peaks eluted before the 9 min mark were due to impurities present in the toluene. Despite extract cleanup, some matrix peaks also were present in the chromatograms. Further cleanup can be possible by increasing the dispersive solid-phase extraction sorbent weight. Nevertheless, all of the 29 pesticides were detected. Calibration, recovery, and precision data are presented in Table II. A first-order fit was used for calibration. Linearity for the five-point calibration curve was excellent with 28 of the 29 pesticides having r2 values greater than 0.995 at a range of 50–500 ng/g.
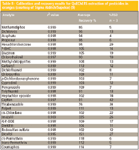
Table II: Calibration and recovery results for QuEChERS extraction of pesticides in oranges (courtesy of Sigma Aldrich/Supelco) (9)
Proper calibration of imazalil was not possible due to its presence in the orange blanks. Several pesticides were tentatively detected in the orange blanks; the identity of imazilil was confirmed spectrally by reanalyzing the sample in the full-scan mode. It was not quantitated because it was outside of the calibration range. Imazilil is a postharvest fungicide commonly used in citrus so it was not surprising to find it in the store-bought sample. Other pesticides also were detected but at low levels; it was suspected that imazalil and dicofol were present because their recovery values were much higher than expected. Overall, recovery and precision were generally good, averaging 102 +/– 13.4% for 27 of the 29 pesticides tested. In the authors' hands, the QuEChERS method proved to be fairly simple and easy to perform.
Other studies performed using QuEChERS have extended the number of pesticides to 229 in fruits and vegetables using GC–MS and LC–MS analysis (10). In this study, recoveries for all but 11 of the analytes were 70–120% (90–110% for 206 pesticides) and repeatabilities typically were less than 10% for a wide range of fortified pesticides in lettuce and orange matrices. Recovery studies by M. Anastassiades (11) at 100 ng/g obtained recoveries of 70–110% with most cases being in the 90–100% range. RSDs fell in the 1–15% range, usually below 5 (n = 5). In his studies, Anastassiades' matrices were apples and cucumbers and he investigated over 250 individual pesticides.
Future of QuEChERS
The QuEChERS approach appears to have a bright future in the analysis of pesticides in foods and other agricultural products. In addition to the references cited previously, the technique has proven successful for the extraction of pesticides from a variety of other fruits and vegetables (12), olives and olive oil (13), barley (14), green beans, peaches, peppers, snow peas (15), strawberries (16), fatty matrices (such as milk, eggs, and avocado) (17) among others. The technique has been used to investigate non-pesticide food toxins such as acrylamide in various food matrices (18) and drugs in blood (19).
There is no reason to believe that QuEChERS could not be used for extractions of other samples besides fruit and vegetable products. For example, Fagerquist and colleagues (20) investigated dispersive-SPE for cleanup of beta-lactam antibiotics in bovine kidney tissue and McMullen and colleagues (21) determined ivermectin in salmon and antioxidants in pet food using QuEChERS. Although there might have to be method adjustments depending upon the analyte–matrix pair, the technique could be adapted to other solid matrices such as solid wastes and other environmental samples, polymeric materials, or just about any other solid that could be homogenized finely and wetted with water. I would expect to see continued application development of this simple, effective, solvent-saving sample preparation technique.
In the European Union (EU), QuEChERS proficiency tests have been conducted every year since between 2002 and 2005 with over 100 laboratories participating each year. These "round robin" tests showed that the method can be transferred among laboratories with success (22). In the United States, the QuEChERS method has been adopted First Action as AOAC International Official Method 2007.01.
Acknowledgments
I would like to thank Steven Lehotay of the U.S.D.A. for providing a number of references and for his careful review of the manuscript with helpful suggestions and to An Trinh of Sigma Aldrich/Supelco and Don Shelly of United Chemical Technologies for providing some background materials from their respective product lines.
Ronald E. Majors
"Sample Prep Perspectives" Editor Ronald E. Majors is business development manager, Consumables and Accessories Business Unit, Agilent Technologies, Wilmington, Delaware, and is a member of LCGC's editorial advisory board. Direct correspondence about this column to "Sample Prep Perspectives," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail lcgcedit@lcgc-mag.com
References
(1) M. Anastassiades, S.J. Lehotay, D. Stajnbaher, and F.J. Schenck, J. AOAC Int. 86, 412–431(2003).
(2) S.J. Lehotay, K. Mastovska, and A.R. Lightfield, J. AOAC Int. 88, 615–629 (2005).
(3) http://www.quechers.com/ (follow link to "QuEChERS — A Mini-Multiresidue Method for the Analysis of Pesticide Residues in Low-Fat Products").
(4) S.A. Barker, J. Chromatogr. A 880, 63–68 (2000).
(5) S.A. Barker, J. Chromatogr. A 885, 115–127 (2000).
(6) S.A. Barker, LCGC, Special Supplement, Review on Modern Solid-Phase Extraction (May 1998).
(7) M. Anastassiades, K. Mastovska, and S.J. Lehotay, J. Chromatogr. A 1015(1-2), 163–184 (2003).
(8) K. Mastovska, S.J. Lehotay, and M. Anastassiades, Anal Chem. 77(24), 8129–8137 (2005).
(9) K. Stenerson, R. Wolford, and O. Shimelis, The Reporter 24(3), 3–5 (2006).
(10) S.J. Lehotay, A. de Kok, M. Hiemstra, and P. Van Bodegraven, J. AOAC Int. 88(2), 595–614 (2005).
(11) M. Anastassiades, http://www.quechers.com/ (follow link to "Recovery Studies using the QuEChERS Method," dated 1/30/04).
(12) F.J. Schenck and J.E. Hobbs, Bull Environ Contam Toxicol. 73(1), 24–30 (2004).
(13) S.C. Cunha, S.J. Lehotay, K. Mastovska, J.O. Fernandes, and M.B.P.P. Oliveira, J. Sep. Sci. in press (2007).
(14) C. Diez, WA Traag, P. Zommer, P. Marinero, and J. Atienza, J. Chromatogr A, 1131 (1–2), 11–23 (2006).
(15) www.flworkshop.com/2003/schenck.pdf.
(16) N. Looser, D. Kostelac, E. Scherbaum, M. Anastassiades, and H. Zipper, Journal fFCr Verbraucherschutz und Lebensmittelsicherheit 1(2), 135–141 (2006)
(17) S.J. Lehotay, K. Mastovska, and S.J. Yun, J. AOAC Int. 88(2), 630–638 (2005).
(18) K. Mastovska and S.J. Lehotay, J. Agric. Food Chem. 54, 7001–7008 (2006).
(19) F. Plossl, M. Giera, and F. Bracher, J. Chromatogr A, 1135(1), 19–26 (2006).
(20) C.F. Fagerquist, A.R. Lightfield, and S.J. Lehotay, Anal.Chem. 77, 1473–1482 (2005).
(21) www.flworkshop.com/2004/schenck25.pdf.
(22) M. Anastassiades, E. Scherbaum, and D. Bertsch, Validation of a Simple and Rapid Multiresidue Method (QuEChERS) and its Implementation in Routine Pesticide Analysis, MGPR Symposium, Aix en Provence, France, May 2003.

Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.
Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








