A Comparison of Two Separation Modes: HILIC and Aqueous Normal Phase Chromatography
LCGC North America
The terms hydrophilic interaction liquid chromatography (HILIC) and aqueous normal phase (ANP) chromatography very often are used interchangeably.
In the literature (1) and in commercial advertising, one can often find material descriptions of chromatographic retention that use the terms hydrophilic interaction liquid chromatography (HILIC) and aqueous normal phase (ANP) in such a manner that they can be construed to be identical. However, in reality, there should be (and there is) a real difference between these two modes of retention and the types of columns that achieve them. A more precise definition of ANP and HILIC is proposed here that will enable chromatographers to distinguish between the two mechanisms and select columns suitable for particular applications.
Experimental
The stationary phases used for this study, UDC cholesterol (75 mm × 4.6 mm) and bidentate C18 (BD C18, 150 mm × 4.6 mm or 20 mm × 2.0 mm), were obtained from MicroSolv Technology (Eatontown, New Jersey). The mobile phase solvents used for chromatographic experiments were acetonitrile (B&J, Muskegon, Michigan) and water purified on a Milli-Q apparatus (Millipore, Bedford, Massachusetts). Formic acid for ANP experiments was obtained from Spectrum Chemical (Gardena, California). The HPLC system used was an Agilent Technologies (Wilmington, Delaware) model 1050 equipped with an autosampler and diode-array detection. Sample concentrations were in the range of 0.1–1 mg/mL with injection volumes of 1–5 μL. The mobile phase for ANP experiments included 0.1% formic acid.
Results and Discussion
HILIC: HILIC is a technique that was designed for the retention and separation of polar–ionic compounds. With the advent of high performance liquid chromatography (HPLC) and the development of reversed-phase separation materials, polar–ionic compounds were found to be eluted at or near the dead volume so that effective analysis of these types of compounds was difficult. Typical reversed-phase materials filled an important need in chromatography when they were developed; retention of hydrophobic compounds and the prevention or reduction of solute adsorption by the silanols on the silica support surface. To induce retention of polar–ionic compounds on the reversed-phase materials, techniques such as solute derivatization (2) or ion-pairing (3) were introduced. In the former method, a chemical reaction converts the polar–ionic compound into a more hydrophobic species while in the latter case, a reagent of opposite charge to an ionic analyte is introduced into the mobile to create a neutral species. These techniques were often cumbersome and time-consuming and thus lengthened the overall analysis time or prevented the use of some modes of detection such as mass spectrometry (MS) or light scattering.
Recent developments in the fabrication of pure silica material have made it more suitable as a stationary phase for the retention of polar compounds (1). The silanols are a polar functional group and, hence, can provide retention for some polar compounds depending upon the choice of mobile phases. Another option is to use standard organosilanization chemistry to modify the surface of the silica, as shown in Figure 1.

Figure 1
If the R group on the organosilane reagent has a polar functionality such as cyano (–CN), amino (–NH2), or diol (–CH(OH)–CH2–OH), then these materials also have an ability to retain certain hydrophilic compounds. Using one of the typical HILIC stationary phases described earlier, the pattern of retention for many polar compounds as a function of mobile phase composition is shown in Figure 2a. At low percentages of organic in the eluent (high water content), there is no retention because the hydrophilic compound prefers to be in the mobile phase. When the mobile phase is sufficiently nonpolar (significant amounts of organic solvent), then retention is observed for many polar compounds. However, as noted on the figure, typical hydrophobic compounds will have little or no retention throughout the entire mobile phase composition range. Thus, while separations of some mixtures of polar compounds is achievable, those samples that contain one or more nonpolar compounds cannot be analyzed effectively because the hydrophobic solutes will be eluted at or near the dead volume over the entire mobile phase composition range.
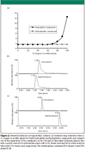
Figure 2
An example of the retention of polar compounds on a HILIC phase is shown in Figure 2c (4). The retention on this silica column is compared to what would be obtained on a typical C18 (reversed-phase) bonded material (Figure 2b). Under these conditions, with only polar species as potential analytes, the HILIC column would be a suitable choice for the separation of compounds such as those shown in Figures 2b and 2c.
ANP: While HILIC often can solve the problem of the retention of polar–ionic compounds, it usually does not fulfill the need to provide an effective separation medium for samples containing a mixture of hydrophilic and hydrophobic species. In fact, it is the opposite of reversed-phase materials, so in either case, the chromatographer usually is faced with a choice of which type of solutes to be retained: hydrophobic ones by using a reversed phase or hydrophilic analytes using a HILIC column. However, some stationary phases do provide retention for both hydrophobic and hydrophilic compounds. Thus, the choice becomes which mechanism will be dominant and the need to distinguish between phases that provide only one mode (reversed phase or HILIC) from those that can have dual hydrophobic–hydrophilic retention properties.
It is with those phases that display a dual mechanism where the term "aqueous normal phase" is most appropriate. The term implies a combination of effects due to interactions that take place because of the aqueous nature of the mobile phase (reversed-phase mechanism) and those that produce retention that mimics a normal phase mechanism (increasing retention as the mobile phase becomes more nonpolar). HILIC only provides the latter and not the former modes. In fact, ANP retention is much more diverse than either simple reversed-phase or HILIC behavior. The following sections describe the various solute elution characteristics that can be encountered in the ANP mode.
ANP 1: Figure 3a illustrates a generic retention map for two solutes (one retained by reversed phase and the other by a normal phase–like mechanism) run on a stationary phase that possess ANP properties. In this case, the two mechanisms clearly are evident, but there is no region on the retention map where the two solutes both have significant retention. Under these circumstances, this phase can be used interchangeably for either reversed-phase or normal phase-like retention depending upon whether a high or low aqueous mobile phase composition is selected. With a high water percentage, the hydrophobic compounds will be retained, while the hydrophilic analytes will be eluted at the void volume. For high organic content mobile phases, the opposite occurs, with the polar compounds being retained and the nonpolar species eluted at the dead volume.
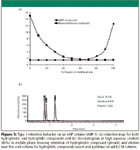
Figure 3
Figure 3b shows the chromatogram of three compounds that exhibit the behavior illustrated in Figure 3a under conditions of high aqueous content in the mobile phase (reversed-phase region). In this case, the ANP stationary phase behaves exactly like a reversed-phase column with retention of hydrophobic compounds and little or no elution beyond the dead volume for very hydrophilic compounds.
ANP 2: Figure 4a shows a second type of retention map that can be obtained for two compounds (one retained by reversed phase and the other by a normal phase-like mechanism) on a phase with ANP properties. In this situation, there is a distinct range of mobile phase compostions in which both compounds display significant retention. Under these conditions, it is possible to separate mixtures of polar and nonpolar compounds, often under isocratic conditions. The other possibility suggested by this retention map is analysis of hydrophobic and hydrophilic compounds using gradient elution with the option of running in either direction, that is, from low to high organic content (standard gradient) or from high to low percent organic (inverse gradient). In contrast to a HILIC phase (only polar compounds retained at high organic compositions) or an ANP 1 situation (retention of the type of compound determined by % organic in mobile phase), the ANP 2 scenario offers unique separation capabilities available from a limited number of commercially available products.

Figure 4
Figure 4b shows an example of the separation of a mixture that illustrates the ANP 2 elution pattern. In this chromatogram, two compounds, one polar (metformin) and one nonpolar (glyburide), are tested on a silica hydride–based C18 column. In the top chromatogram at 50:50 acetonitrile–water, the reversed-phase mechanism is dominant and glyburide is retained more strongly than the polar compound metformin. The middle chromatogram is obtained when the mobile phase has been changed to 80:20 acetonitrile–water. Under these conditions, the normal phase-type mechanism has become slightly stronger than the reversed-phase mode, and metformin is eluted just after glyburide (confirmed by LC–MS). If the acetonitrile content is increased further (85:15), then the normal phase-type mechanism becomes dominant, and metformin is retained significantly longer than glyburide.
ANP 3: A third possibility also can be obtained on a true ANP stationary phase. This situation is illustrated by the generic retention map shown in Figure 5a. This is an elution pattern for certain types of compounds; those that have both hydrophobic and hydrophilic properties. These solutes are typically larger molecules with one or more polar functionalities as well as a significantly hydrophobic portion. Some peptides and proteins are examples of molecules that fit this description. Under these circumstances, the chromatographer has a choice of operating in the reversed-phase or ANP mode depending upon the types of other molecules in the mixture, sample compatibility with the mobile phase, or the means of detection utilized. This unusual capability provides experimental flexibility that is not available on typical stationary phases fabricated through the use of organosilane chemistry.
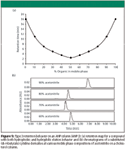
Figure 5
Figure 5b provides an example of retention of a compound that is retained by both a reversed-phase and normal phase-type mode depending upon the mobile phase composition selected. The series of chromatograms shown follows the retention profile predicted by the map in Figure 5a. As expected, retention goes through a minimum with increasing acetonitrile concentration, indicating that the mechanism is changing from reversed phase to ANP.
Stationary phases exhibiting ANP retention: Recently, it has been demonstrated that stationary phases based upon a hydride surface (commercially known as TYPE-C Silica, MicroSolv Technology) display all three of the ANP properties described earlier and the columns used for the examples in Figures 3b, 4b, and 5b are based upon this material (5,6). The exact difference in the surface composition between ordinary silica and hydride silica is in Figure 6 (hydride surface coverage is 95%).
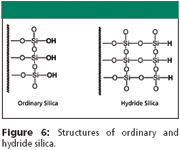
Figure 6
At present, a detailed mechanism responsible for the ANP behavior is not developed completely. Some of the other properties of silica hydride-based stationary phases are the ability to operate reproducibly in the organic normal phase without the need to carefully eliminate water from the mobile phase solvents, to function under conditions of 100% aqueous eluents without phase collapse, and to reequilibrate after a gradient (standard or inverse) within 1 min. These properties are directly in contrast to those of a HILIC material, in which water is assumed to form on layer on the support surface (1). On the hydride surface, because of its more hydrophobic nature, little if any water is expected to be present. Thus, water washes of the stationary phases with ANP properties are not required regularly, as they are with a HILIC phase. Investigations are ongoing using both spectroscopic and chromatographic methods to elucidate the exact nature of the solvated surface and solute–hydride interactions that would shed some light on ANP behavior and other properties of the hydride-based stationary phases.
Conclusions
HILIC was designed for and provides interesting retention of polar compounds. Silica hydride-based columns using the ANP mode of chromatography are designed for long-term use in retaining both polar and nonpolar compounds. These columns can be very useful for complex mixtures containing both hydrophobic and hydrophilic compounds. The dual retention capabilities allow an ANP column to operate over a broad range of mobile phase compositions with the unifying factor being the presence of water in the mobile phase. While some stationary phases (like unendcapped organosilane materials) can retain both polar and nonpolar compounds, the applications usually are limited and often are either not satisfactory or reproducible. However, other synthetic strategies in the future can also result in bonded phases with the same or similar properties as those of the hydride-based columns.
Joseph Pesek and Maria T. Matyska
Department of Chemistry, San Jose State University, San Jose, California
Please direct correspondence to Joseph Pesek at pesek@sjsu.edu
References
(1) P. Hemstrom and K. Ingrum, J. Sep. Sci. 29, 1784–1821 (2006).
(2) S. Soback, J. AOAC Int. 82, 1017–1045 (1999).
(3) B.B. Ba and M.-C. Saux, J. Chromatogr. B 764, 349–362. (2001).
(4) K.M. Peru, S.L. Kuchta, J.V. Headley, and A.J. Cessna, J. Chromatogr. A 1107, 152–158 (2006).
(5) J.J. Pesek and M.T. Matyska, LCGC 24(3), 296–303 (2006).
(6) J.J. Pesek and M.T. Matyska, J. Sep. Sci. 30, 637–647 (2007).

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.














