Quadrupole Mass Analysers: An Introduction
An excerpt from LCGC's e-learning tutorial on quadrupole mass analysers at CHROMacademy.com
Quadrupole mass analysers were first described and developed in 1953 by the West German physicists Wolfgang Paul and Helmut Steinwedel while they were working at the University of Bonn. Electric fields are used to separate ions according to their mass-to-charge ratio (m/z), the ratio of mass in daltons (Da) to the integer number of charges (z), as they pass along the central axis of parallel and equidistant poles or rods. Each rod has two voltages applied, one of which is a fixed direct current and the second is an alternating current that cycles with a superimposed radio frequency (10 kHz is not uncommon).
The magnitude of the applied electric field can be ordered such that only ions with a specific m/z ratio can travel through the quadrupole, prior to being detected. Ions with all other m/z values are deflected onto trajectories that would cause them to collide with the quadrupole rods and discharge, or be ejected from the mass analyser field and removed via the vacuum. The quadrupole is often referred to as an exclusive detector because only ions with a specific m/z are stable in the quadrupole at any one time. Those ions with a stable trajectory are often referred to as having noncollisional, resonant or stable trajectories.
A typical quadrupole mass analyser consists of four rods with a hyperbolic cross section. The quadrupole rods are typically constructed using molybdenum alloys because of their inherent inertness and lack of activity. Very high degrees of accuracy and
precision (in the micrometre region) in rod machining and relative positioning are required to achieve unit mass accuracy (Figure 1). For clarity the figure shows the rods with a much smaller diameter than in reality.
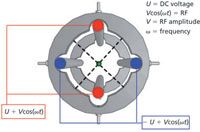
Figure 1: Schematic diagram showing the construction and applied voltages for a typical quadrupole mass analysing device.
It is a common misconception that the quadrupole mass analyser consists of a pair of positive and negative rods. Because of the voltage oscillating at a radio frequency (commonly known as the "RF voltage"), each pair of rods will be successively positive then negative and so forth. In essence, there will always be a pair of positive and negative rods; however, they will alternate at the radio frequency. The misnomer arises as one rod pair has a negative direct current (DC) voltage offset (-U) and the other a positive DC offset voltage (+U).
An ion traveling through the quadrupole will successively be attracted and then repelled from each rod until it reaches what is known as a "saddle" field.
At certain values of U and V (DC and RF as they are colloquially referred to), ions of a particular mass-to-charge ratio will oscillate with a trajectory that is "within" the space between rods (often called the "tunnel radius"). When this occurs, the ion, which is also accelerated through the mass analysing device with an applied voltage between the two quadrupole ends, reaches the detector. The relationship between the DC and alternating current (RF) voltages and the mass-to-charge ratio of stable ions can be plotted on a Mathieu diagram.
Quadrupole rods may be "tuned" using a compound that reproducibly fragments to give ions of particular mass-to-charge ratio. A popular compound for tuning the quadrupole in electron ionization gas chromatography–mass spectrometry (GC–MS) is perfluorotributylamine, which fragments very reproducibly over a wide mass range with relative fragment intensities that are known and reproducible. The magnitude of the applied voltages to allow the passage of specific fragment ions can then be assigned and the "mass axis" can be calibrated. Furthermore, the DC and RF voltages can be adjusted to alter the resolution and sensitivity of the device. This operation is typically carried out using an automated instrument algorithm, although learning to tune the device manually can result in much higher sensitivity for particular target ion masses.
Typically, a single spectral experiment involves a range of DC to RF values being scanned to sequentially allow ions across the full mass range to pass through the analyser. This is called a "scan" function, and a scanning rate of 10 Hz is considered very good across a mass range of 50–500 m/z. The total ion abundance of each successive scan is summed to produce a total ion current (TIC), which functions as the pseudo chromatogram — of course the contributing spectrum for each data point can then be extracted if required.
For higher sensitivity, the scan range of the quadrupole can be limited or fixed to certain DC to RF values that correspond to known high abundance ions produced by the analyte of interest. In this way, the frequency of measurement is increased and consequently the signal-to-noise ratio increases; this is known as selected ion monitoring.
Typical commercial quadrupole instruments can achieve unit mass resolution; that is, mass 201 and mass 202 can be sufficiently resolved from each other, although under ideal conditions the quadrupole is capable of much higher resolution. Commercially available instruments typically achieve resolution values of up to 5000 (full width half maximum), with mass accuracy of higher than 100 ppm, meaning that automated derivation of elemental composition is not possible. Single-quadrupole liquid chromatography LC–MS and GC–MS instruments are used in laboratories all over the world and provide a cost effective, but relatively sensitive and selective means of ion detection.

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.
A Guide to (U)HPLC Column Selection for Protein Analysis
April 16th 2025Analytical scientists are faced with the task of finding the right column from an almost unmanageable range of products. This paper focuses on columns that enable protein analysis under native conditions through size exclusion, hydrophobic interaction, and ion exchange chromatography. It will highlight the different column characteristics—pore size, particle size, base matrices, column dimensions, ligands—and which questions will help decide which columns to use.











