An Improved LC–MS–MS Method for the Determination of Clenbuterol in Human Urine
LCGC Europe
An improved liquid chromatography tandem mass spectrometry (LC–MS–MS) method for the screening and confirmation of clenbuterol in human urine in sports doping applications was developed and validated.
An improved liquid chromatography tandem mass spectrometry (LC–MS–MS) method for the screening and confirmation of clenbuterol in human urine was developed and validated in this study. In addition to the currently used ion transitions (277.1→203.0 and 277.1→259.1), two new ion transitions (277.1→132.0 and 277.1→168.0) were utilized for this new screening procedure. Compared with existing methods of clenbuterol screening in human urine, the developed method is of higher sensitivity and reduces the number of false-positive results. The limit of detection (LOD) of the method proposed is 2 pg/mL, which is lower than the minimum required performance limit (MRPL) set by World Anti-Doping Agency (WADA). The success and reliability of the method in doping control analysis was demonstrated for the analysis of a 2011 EQAS (External Quality Assessment Systems) -01 human urine sample obtained from WADA.
Clenbuterol (Figure 1) is a beta-2 adrenergic agonist that stimulates beta-2 adrenergic receptors resulting in relaxation of smooth muscle and dilatation of bronchus, widely used to treat asthma and respiratory disease (1–3). A novel effect of clenbuterol is to slightly increase body temperature, increasing fat metabolism and magnifying the effect of anabolic steroids taken simultaneously (4,5). Therefore, many athletes use clenbuterol to increase their blood pressure and stimulate the heart muscle to maximize their potential. In 2011, clenbuterol was classified within "other anabolic steroids" and listed as prohibited in athletics by the World Anti-Doping Agency (WADA). The minimum required performance limit (MRPL) in and out of competition was set at 2 ng/mL in human urine (6). Following this classification, many publications reported on the two main clenbuterol detection methods: chromatography and immunoassay. Chromatographic techniques to determine clenbuterol include gas chromatography–mass spectrometry (GC–MS) (7–9), high-performance liquid chromatography (HPLC) (10,11) and liquid chromatography–mass spectrometry (LC–MS) (12,13). Immunoassay methods include radio-immunoassay, competitive fluorescence immunoassay and enzyme-linked immunosorbent assays that provide relatively cheap and simple analysis approaches (14–16); however, these immunoassays cannot provide structural information of the molecule, hence they are only suitable for complementary detection.

GC–MS and LC–MS are the two methods currently approved by the WADA for use in doping control, with both the chromatographic retention time and diagnostic mass spectrometric ions required for the identification of a compound (17). However, as GC–MS (or MS–MS) requires time-consuming derivatization steps to enhance the volatility of analytes, liquid chromatography tandem mass spectrometry (LC–MS–MS) has become the main analytical technique for the determination of clenbuterol because of its shorter chromatographic run time and the elimination of time-consuming derivatization procedures.
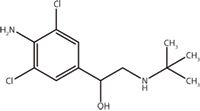
Figure 1: The chemical structure of clenbuterol.
Many LC–MS–MS methods have been developed for identification of clenbuterol (18–27), but there are few papers on the identification of clenbuterol in human urine. An LC–MS–MS method for determining clenbuterol in equine and human urine has been developed by Mario et al. (28). The pre-treatment procedure included acidic hydrolysis, neutralization and weak basic extraction. The limits of detections (LODs) were 2 ng/mL (human urine) and 10 ng/mL (equine urine), respectively. An LC–MS–MS approach was also reported for the detection of clenbuterol in human urine by using a three-phase solvent bar microextraction (TPSBME) technique for sample pre-treatment (29) and the reported LOD was 7 pg/mL.
It is worth noting that all the reported LC–MS–MS methods for the characterization of clenbuterol employed two abundant transitions at m/z = 277→259 and 277→203 (18–29). These transitions may produce false positive results and so the reported LODs are unreliable. The aim of this study is to develop and validate a more sensitive and specific method for the screening and confirmation of clenbuterol in human urine for doping control purposes.
Experimental
Chemicals and Reagents: All solvents (HPLC grade) were obtained from Dima Tech. Inc. (California, USA). Sodium dihydrogen phosphate monohydrate, disodium hydrogen phosphate dehydrate, hydrochloric acid and potassium hydroxide were purchased from Sinopharm Chemical Reagent Co. Ltd (Beijing, China). E. coli β-Glucuronidase was obtained from Sigma-Aldrich (Shanghai, China). Clenbuterol and clenbuterol-d9 (the internal standard for confirmation) were purchased from Shanghai ANPL Scientific Instrument Co., Ltd (Shanghai, China). The standard stock solution of clenbuterol was prepared in methanol (1 mg/mL). 17α-Methyltestosterone (the internal standard for screening) was purchased from Sigma-Aldrich (Missouri, USA). Two internal standard (IS) solutions (1 ng/mL for clenbuterol-d9 and 10 µg/mL for 17α-methyltestosterone) were also prepared in methanol.
Sample Preparation
Pre-treatment for Screening: Two millilitres of urine was added in a glass tube and buffered to pH 7.0 with 1 mL of 0.8 M phosphate buffer (Na2HPO4:NaH2PO4, 1:2, w:w). Fifty microlitres of β-Glucuronidase from E.coli and 20 µL of 17α-methyltestosterone solution were added and the sample was then heated at 55 °C for 1 h. After cooling to ambient temperature, 0.5 mL aqueous solution containing potassium carbonate and potassium bicarbonate (20%, 1:1, w:w) plus 4 mL of methyl tert-butyl ether were added. Subsequently, the mixture was shaken mechanically for 10 min and then centrifuged for 5 min. The organic layer was transferred to a fresh glass tube, flowed to dryness at 25 °C and the dry residue was dissolved with 500 µL mixture consisting of 10 mM aqueous ammonium formate buffer (which was adjusted to pH 3.5 with formic acid) and acetonitrile containing 0.5% formic acid [90:10 (V/V)]. Twenty microlitres of the reconstituted solution was injected into the LC–MS–MS for instrument analysis.
Pre-treatment for Confirmation: To obtain better sensitivity, the strong acidic extraction and neutralization steps were incorporated into the pre-treatment procedures of confirmation to remove the acidic and neutral interfering substances. Four hundred microlitres of 1 M hydrochloric acid and 2 µL of clenbuterol-d9 solution were added into 2 mL of urine. Subsequently, 4 mL of methyl tert-butyl ether was added, the mixture was shaken for 10 min then centrifuged at 2500 rpm for 5 min. The organic layer of the centrifuge solution was discarded and 0.2 mL of 5 M aqueous KOH and 4 mL of methyl tert-butyl ether were added into the remaining aqueous phase. The mixture was shaken for 10 min and centrifuged at 2500 rpm for 10 min. The organic layer of the centrifuge solution was transferred to a fresh test tube and evaporated to dryness at 40 °C under vacuum. The dry residue was dissolved in a 250 µL mixture consisting of 10 mM aqueous ammonium formate buffer (adjusted to pH 3.5 by the addition of formic acid) and acetonitrile containing 0.5% formic acid [90:10 (V/V)]. 20 µL of the reconstituted solution was injected into the LC–MS–MS system for analysis.
Instrument Parameters: All analyses were performed on an triple-quadrupole 6410 LC-MSD (Agilent, Waldbronn, Germany) equipped with binary pump, on-line vacumn degasser, autosampler, column compartment, mass spectrometer detector of electrospray interface and ChemStation (Agilent, Santa Clara, California, USA). The LC was equipped with a Zorbax XDB-C18 column (50 mm length, 2.1 mm inner diameter, 3.5 µm particle size) (Agilent) linked to a filter (particle size 0.2 µm) and the separation was performed at constant temperature (40 °C).
The mobile phase was composed of 10 mM aqueous ammonium formate buffer (which was adjusted to pH 3.5 with formic acid) together with acetonitrile [90:10 (V/V)] (eluent A). Eluent B was pure acetonitrile. A gradient was utilized starting at 10% eluent B and increasing to 90% eluent B within 7 min. The column was flushed for 1 min at 100% eluent B and finally re-equilibrated at 10% eluent B for 3 min. The flow rate was set at 0.5 mL/min. Multiple reaction monitoring (MRM) mode was used to measure the drug in positive ionization (PI) mode. Product ion scan experiments were performed using collision-induced dissociation (CID) with nitrogen as the collision gas at 5.33e-3 Pa (obtained from a nitrogen generator, (CMC, Eschborn, Germany). "Wide" resolution (for screening procedure) and "Unit" resolution (for confirmation procedure) were used for mass selection in MS1 and MS2. The spray voltage was set at 4.0 kV. The ion source was operated at 330 °C. Nitrogen was also used as a nebulizing and drying gas, with flow and nebulizer pressure set at 10.0 mL/min and 35 psi, respectively.

Figure 2: The extracted ion chromatogram from (a) full MS scan and (b) mass spectra of clenbuterol (fragmentor: 100 V; B: the characteristic chlorine isotope pattern was shown).
Results and Discussion
Selection of Ion Transition: As a nitrogen-containing compound, clenbuterol was liable to protonate under positive ion ESI conditions. The protonated ion [M+H]+ at m/z = 277.1 was yielded in full scan mode with the characteristic chlorine isotope pattern (Figure 2). The MS behaviour of the m/z = 277.1 was investigated carefully in product ion scan mode. Product ion scan of the m/z = 277.1 at the low level of collision energy (5–15 eV) produced the m/z = 259.1 ion which originated from the neutral loss of a water molecule (-18 µ) (Figure 3), but the most abundant product ion resulted from the consecutive elimination of isobutene (-56 µ) giving rise to the ion at m/z = 203.0. The product ions at m/z = 168.0, 132.0, 140.0 and 57.1 were accordingly produced with the level of collision energy increased from 10 eV to 40 eV (Figure 4). Nevertheless, the four product ions showed much lower abundance than the m/z = 203.0 and 259.1 and the m/z = 140.0 ion has not been used yet.
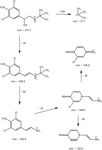
Figure 3: Possible fragmentation pathway of clenbuterol (m/z = 140.0 ion has not been reported before).
The specificity of the six transitions (277.1→203.0, 277.1→259.1, 277.1→132.0, 277.1→168.0, 277.1→140.0 and 277.1→57.1) in human urine were specifically studied. Interestingly, the three transitions comprised of the even product ions (m/z = 168.0, 132.0 and 140.0) displayed more specificity than those of the odd product ions at m/z = 259.1, 203.0 and 57.1. In LC–MS–MS qualitative analysis, the most important issue is the specificity of the detection. The screening approaches included at least two transitions for clenbuterol in order to increase the specificity of the method. However, the selection of the two most abundant transitions doesn't always ensure the specificity of the method in human urine. The presence of some endogenous interfering substances with a similar structure in the urinary matrix could reduce the specific detection of clenbuterol. For this purpose, the selection of a specific transition was of utmost importance. Therefore, the two transitions at m/z = 277.1→168.0 and 277.1→132.0 were used for screening clenbuterol in human urine under positive ion MRM mode and confirmatory analysis was accomplished by employing the transitions at m/z = 277.1→168.0, 277.1→132.0 and 277.1→140.0 combined with the improved pre-treatment approach, as presented in the experimental section.

Figure 4: Product ion mass spectra of clenbuterol at the collision energy of 15 eV (a) and 40 eV (b) (the abundant product ion was the m/z = 203.0, and the product ions at m/z = 168.0, 132.0, 140.0 and 57.1 were accordingly produced with the level of collision energy grown up from 15 eV to 40 eV).
Method Evaluation: The LOD was defined as the lowest level that could be calculated with the diagnostic ions present at a signal-to-noise ratio (S/N) greater than three. Seven blank human urine samples were spiked with the target substances at eight different concentrations of 1 pg/mL, 2 pg/mL, 5 pg/mL, 10 pg/mL, 20 pg/mL, 50 pg/mL, 100 pg/mL and 200 pg/mL and analysed according to the established protocol to estimate the LODs (Table 1). The LODs of screening and confirmation methods were 50 pg/mL and 2 pg/mL (Figure 5), respectively.

Table 1: Evaluation of the developed LCâMSâMS method for the determination of clenbuterol in urine.
Six blank human urine specimens were spiked with the target substances prior to sample preparation and another six blank human urine samples were extracted according to the protocol described above. This was then followed by the addition of the target substances into the final extracts. The IS solution was spiked into the extracts of the samples before evaporation. Recoveries were calculated by comparison of mean peak area ratios of clenbuterol and the IS spiked prior to and after extraction; the recoveries of screening and confirmation were 86.2% and 95.5%, respectively (Table 1). Twenty different human urine specimens (10 males and 10 females), declared negative after routine doping analysis, were pre-treated as described above and no interfering peaks were found in the selected ion transition chromatograms at the expected retention time of the analyte. Interday (n= 18) and intraday (n = 6) precisions were less than 4.2% and 9.3% at the spiked concentration of 0.5 ng/mL.
Method Comparison: The developed LC–MS–MS method (transitions at m/z = 277.1→168.0 and 277.1→132.0) for screening clenbuterol in human urine was compared to a currently used LC–MS–MS method which uses two ion transitions at m/z = 277.1→259.0 and 277.1→203.0 for quantification. Figure 6(a) and (b) are the overlapped MRM chromatograms of a negative urine sample using two methods. It clearly showed that the new method can provide more accurate results and reduce the number of false positive results compared to when traditional ion transitions was used for quantification.

Figure 6: The overlapped MRM chromatograms (a) and (b) of a negative urine sample for screening; (a): extracting the m/z = 277.1â203.0 and 277.1ï¢â259.0 transitions, many endogenous interfering substances containing these two transitions appeared at the expected retention time of clenbuterol; (b): extracting the m/z = 277.1âï¢132.0 , 277.1âï¢168.0 and 277.1âï¢140.0 transitions, no interfering substances containing these three transitions appeared at the expected retention time of clenbuterol, the false positive of clenbuterol may be given by using the m/z = 277.1ï¢â203.0 and 277.1ï¢â259.0 transitions in screening procedure.
Methodology Validation: The validated procedure was applied to a 2011 External Quality Assessment Systems (EQAS)-01 sample from WADA and an adverse analytical finding (AAF) for clenbuterol was produced in screening LC–MS–MS measurement. Figure 7(b) showed that a co-eluting endogenous interfering peak with the same protonated ion at m/z = 277.1 was presented in total ion chromatogram (TIC) of a blank urine specimen, but no ion transitions at m/z = 277.1→168.0 and 277.1→132.0 were found [Figure 7(c) and (d)]. The presence of clenbuterol in both 2011 EQAS-01 sample and clenbuterol spiked blank urine sample were unambiguously confirmed using three diagnostic ion transitions as illustrated in Figure 7(e) and (f). Retention times and relative abundances of peaks obtained from selected ion transitions were all within the limits established by the WADA.

Figure 7: The screening and confirmation chromatograms (aâf) of the 2011 EQAS-01 sample (a) MRM chromatogram for screening; (b) the total ion chromatogram of a blank urine sample, a co-eluting endogenous interfering peak with the same protonated ion at m/z = 277.1 was presented; (c) the extracted transition at m/z = 277.1âï¢132.0 of the blank urine; (d) the extracted transition at m/z = 277.1âï¢168.0 of the blank urine; (e) the three extracted transitions at m/z = 277.1ï¢â130, 277.1âï¢168.0 and 277.1âï¢140.0 of the 2011 EQAS-01 sample; (f) three transitions at m/z = 277.1ï¢â130, 277.1âï¢168.0 and 277.1âï¢140.0 of a blank urine spiked with 5 ng/mL of clenbuterol.
Conclusions
A two-step method for the screening and confirmation of clenbuterol based on an LC–MS–MS technique has been developed and validated, addressing the requirements of the minimum required performance limit (MRPL) established by the WADA. Identification of the drug is initially based on its retention time and two specific ion transitions at m/z = 277.1→132.0 and 277.1→168.0 under positive ESI condition in MRM mode and subsequently confirmed by comparison of the relative intensity of three characteristic ion transitions (m/z = 277.1→132.0, 277.1→168.0 and 277.1→140.0) under positive ion ESI conditions. This approach, together with the improved pretreatment procedures, is a powerful analytical technique with a high degree of specificity and sensitivity, compared with the existing LC–MS–MS method in which two abundant ion transitions (277.1→203.0 and 277.1→259.1) were used to identify clenbuterol in human urine.
Acknowledgement
This research was supported by National Natural Science Foundation of China (20877103, 21077135).
Jianghai Lu is a research fellow of the National Anti-Doping Laboratory, China Anti-Doping Agency. He obtained his PhD at Peking University in 2001 and has been engaged in doping control analysis up to now.
Gangfeng Ouyang is a professor of chemistry at the School of Chemistry and Chemical Engineering, Sun Yatsen University, China. He received his PhD in physical chemistry from Sun Yat-sen University in 2003. His postdoctoral work was in analytical chemistry at the University of Waterloo, Canada. He is an author of over 70 scientific publications, a member of the editorial board of Analytica Chimica Acta and Microchemical Journal, and special issue guest editor of Trends in Analytical Chemistry.
References
(1) A.R. Martin, J.N. Delgado and W.A. Remers (Eds.), Textbook of Organic Medicinal and Pharmaceutical Chemistry, Lippincott, Philadelphia, Pennsylvania, USA, (1991).
(2) H.S. Nelson, New Engl. J. Med., 333(8), 499–506 (1995).
(3) M. Nishikawa, J.C. Mak and P.J. Barnes, Eur. J. Pharmacol., 318(1), 123–129 (1996).
(4) C. Culmsee, R.K. Stumm, M.K.H. Schafer, E. Weihe and J. Krieglstein, Eur. J. Pharmacol., 379(1), 33–45 (1999).
(5) I.D. Prather, D.E. Brown, P. North and J.R. Wilson, Med. Sci. Sport. Exer., 27(8), 1118–1121 (1995).
(7) L. Amendola, C. Colamonici, R. Cristiana and F. Botre, J. Chromatogr. B, 773(1), 7–16 (2002).
(8) M.D. Engelmann, D. Hinz and B.W. Wenclawiak, Anal. Bioanal. Chem., 375(3), 460–464 (2003).
(9) I. Garcia, L. Sarabia, M.C. Ortiz and J.M. Aldama, Anal. Chim. Acta, 515(1) 55–63 (2004).
(10) G. Brambilla, M. Fiori, B. Rizzo, V. Crescenzi and G. Masci, J. Chromatogr. B, 759(1) 27–32 (2001).
(11) A. Blomgren, C. Berggren, A. Holmberg, F. Larsson, B. Sellergren and K. Ensing, J. Chromatogr. A, 975(1), 157–164 (2002).
(12) C. Crescenzi, S. Bayoudh, P.A.G. Cormack, T. Klein and K. Ensing, Anal. Chem., 73(10) 2171–2177 (2001).
(13) J. Blanca, P. Munoz, M. Morgado, N. Mendez, A. Aranda, T. Reuvers and H. Hooghuis, Anal. Chim. Acta, 529(1–2) 199–205 (2005).
(14) S.A. Haughey, G.A. Baxter, C.T. Elliot, B. Persson, C. Jonson and P. Bjurling, J. AOAC Int., 84(4), 1025–1030 (2001).
(15) A. Roda, A.C. Manetta, F. Piazza, P. Simoni and R. Lelli, Talanta, 52(2), 311–318 (2000).
(16) I.K. Abukhalaf, D.A. von Deutsch, B.A. Parks, L. Wineski, D. Paulsen, H.Y. Aboul-Enein and D.E. Potter, Biomed. Chromatogr., 14(2), 99–105 (2000).
(18) C. Li, Y.L. Wu, T. Yang, Y. Zhang and W.G. Huang-Fu, J. Chromatogr. A, 1217(50), 7873–7877 (2010).
(19) C. Juan, C. Igualada, F. Moragues, N. Leon and J. Manes, J. Chromatogr. A, 1217(39), 6061–6068 (2010).
(20) R.M. Costain, A.C.E. Fesser, D. McKenzie, M. Mizuno and J.D. MacNeil, Food Addit. Contam. A, 25(12), 1520–1529 (2008).
(21) A. Wen, T. Hang, S. Chen, Z. Wang, L. Ding, Y. Tian, M. Zhang and X. Xu, J. Pharmaceut. Biomed. Anal., 48(3), 829–834 (2008).
(22) H. Geyer, M.K. Parr, K. Koehler, U. Mareck, W. Schanzer and M. Thevis, J. Mass Spectrom., 43(7), 892–902 (2008).
(23) M.K. Parr, K. Koehler, H. Geyer, S. Guddat and W. Schanzer, Biomed. Chromatogr.,22(3), 298–300 (2008).
(24) G.N.W. Leung, D.K.K. Leung, T.S.M. Wan and C.H.F. Wong, J. Chromatogr. A, 1156(1–2), 271–279 (2007).
(25) B. Yang, J.B. Zhu, C.H. Deng and G.L. Duan, Rapid Commun. Mass Sp., 20(12), 1883–1186 (2006).
(26) H.L. Li, W.D. Zhang, R.H. Liu, C. Zhang, T. Han, X.W. Wang, X.L. Wang, J.B. Zhu and C.L. Chen, J. Chromatogr. B, 831(1–2), 140–146 (2006).
(27) A.C.E. Fesser, L.C. Dickson, J.D. MacNeil, J.R. Patterson, S. Lee and R. Gedir, J. AOAC Int., 88(1), 61–69 (2005).
(28) M. Thevis, G. Opfermann and W. Schanzer, J. Mass Spectrom.,38(11), 1197–1206 (2003).
(29) M.B. Melwanki, S.D. Huang and M.R. Fuh, Talanta, 72(2), 373–377 (2007).

Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.








