Enantiomer and Topoisomer Separation of Acidic Compounds on Anion-Exchanger Chiral Stationary Phases by HPLC and SFC
LCGC Europe
Quinine- and quinidine-derived anion-exchanger chiral stationary phases have proven to be versatile in enantiomer separation of acidic compounds in HPLC. In this article, the authors demonstrate their performance in specific HPLC applications involving enantiomer resolution and topoisomer separation.
Quinine- and quinidine-derived anion-exchanger chiral stationary phases are versatile tools for enantiomer separation of acidic compounds in high performance liquid chromatography (HPLC). This article demonstrates their recognition ability in specific HPLC applications, involving enantiomer resolution and plasmid topoisomer separation. The extension of their applications from HPLC to supercritical fluid chromatography (SFC) was also investigated, with the aim of assessing the influence of a series of parameters and gaining insight into the general approaches for SFC method development and optimization.
Quinine (QN) and quinidine (QD) are alkaloids of the Cinchona family with anti-malarial properties that have a long tradition in stereoselective methods as auxiliaries (as a base for fractionated crystallization of chiral acids), as chiral catalysts and as chiral selectors for enantioselective separations. Based on the investigations of Lindner and his fellow workers, the chiral recognition ability of various derivatives has been explored recently for the resolution of acidic enantiomers. Most notably, it was found that a carbamoyl modification of the secondary hydroxyl group at C9 of the alkaloid significantly enhanced the enantiorecognition capabilities of the resulting chiral selector (1-3). The tert-butyl carbamates of QN and QD immobilized on spherical silica gel (Figure 1) turned out to be the most versatile compromise of structure variations. When used with weakly acidic mobile phases — usually pH 4–7 — they act as weak anion exchanger chiral stationary phases (CSPs) to provide specific enantioselectivity for acidic compounds.

The enantiomer recognition mechanism is based on ionic interaction between the protonated tertiary nitrogen of the quinuclidine moiety of the chiral selector and the anionic analytes. Such an ion pairing is accompanied by additional intermolecular interactions including hydrogen bonding, dipoledipole, p–p and hydrophobic, as well as steric interactions (2–4).
These chiral columns have been exhaustively investigated in HPLC with aqueous and non-aqueous polar organic mobile phases and show remarkable performance in enantiomer resolution of a wide variety of acidic compounds (3–10) and so investigation of these columns for enantiomer separation by SFC is a current area of research.
In this article we demonstrate that the application of these columns can be extended to different practical applications in LC and SFC for enantiomer resolution of acidic compounds and for separation of certain specific compounds like topoisomers of circular plasmid DNA.
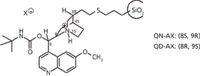
Figure 1: The chiral stationary phases.
Experimental
Columns: Commercially available Chiralpak QN–AX and Chiralpak QD–AX are products of Daicel Group (Tokyo, Japan), manufactured at Chiral Technologies Europe (Illkirch, France). Within the text they are referred to as QN AX and QD AX columns. The columns packed with the 5 µm CSPs had a 4.6 × 100 mm selected dimension for all the SFC experiments; and a 4.0 × 150 mm dimension for the HPLC investigation of lactic acid and a 4.0 × 100mm dimension for the HPLC study on plasmid DNA topoisomer separation.
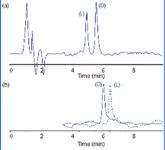
Figure 2: HPLC enantiomer separations of D- and L-lactic acid on QN AX and QD AX columns. (a) Mixture of D- and L-lactic acid (60:40; w/w) on QN AX. (b) Reversal of elution order on QD AX. Experimental conditions: mobile phase: methanol-acetonitrile (50:50; v/v) containing 30 mM formic acid adjusted to apparent pH 4 (with ammonia); temperature: 15 °C; flow rate: 1 mL/min; UV detection at 230 nm.
Lactic Acid Enantiomer Separation: HPLC experiments were carried out using a 1100 series LC (liquid chromatography) system from Agilent Technologies (Waldbronn, Germany) equipped with a binary gradient pump, autosampler, autosampler thermostat (temperature set at 5 °C), vacuum degasser and a temperature-controlled column compartment. D- and L- lithium lactate were purchased from Sigma–Aldrich.
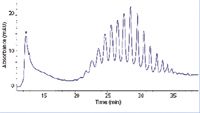
Figure 3: Chromatographic separation of distinct supercoiled plasmid DNA topoisomers and open-circular form on QN AX. Chromatographic conditions: mixed NaCl and 2âpropanol gradient from 0 to 100% B in 60 min. Eluent A: 50 mM NaH2PO4 adjusted to pH 7.0 with 5 M NaOH. Eluent B: 50 mM NaH2PO4, 0.6 M NaCl and 10% (v/v) 2-propanol adjusted to pH 7.0 with 5 M NaOH. Between injections the column was regenerated by a salt plug (injection of 50 µl 3 M NaCl [aq.]), followed by re-equilibration at 0% B for 5 min. Flow rate: 0.7 mL/min; UV-detection at 258 nm; column temperature: 60 °C with preheating. The asterisk denotes the oc isoform.
LC–tandem mass spectrometry (LC–MS–MS) experiments were performed on an Agilent 1200 HPLC system (Waldbronn, Germany) coupled to a QTrap 4000 (Applied Biosystems/MDS Sciex, Ontario, Canada). The HPLC system was equipped with a thermostated autosampler (that allowed cooling samples to 5 °C, a binary pump and a column thermostat. The use of a turbo-ionspray interface allowed splitless hyphenation of HPLC (1 mL/min) with MS. Data sets were processed using the analyst 1.4.1. software from MDS Sciex (San Francisco, California, USA). The experiments were carried out in SRM mode monitoring the pseudo-SRM (selected reaction monitoring) transition of m/z = 89 as precursor and product ion for quantification, and the SRM transition with m/z = 89 as precursor ion and m/z = 43 as qualifier transition.
Plasmid DNA Topoisomer Separation: HPLC analyses were carried out on a 1200 SL Rapid Resolution system (Agilent) equipped with an autosampler thermostat (4 °C) and a DAD UV detector (Agilent Technologies, Waldbronn, Germany). pMCP1 (4.9 kbp, 2.8 mg/mL) plasmid DNA in Tris EDTA buffer was provided by Boehringer- Ingelheim RCV (Vienna, Austria), with homogeneities [covalently closed circular (ccc) form contents] > 90%. The chromatographic conditions are specified in the caption of Figure 3.
SFC Separations:The carbon dioxide (CO2) supply was from a cylinder of B50 of industrial quality 4.8. Methanol (MeOH) of HPLC quality was used as the bulk modifier of the supercritical fluid carbon dioxide (SF-CO2). Formic acid (FA) or acetic acid (HOAc) was employed as the acidic additive. They were matched respectively by ammonium formate (NH4OOCH) and ammonium acetate (NH4OAc) to balance the analyte retention times. The acidic compounds from Sigma-Aldrich (Figure 4) were dissolved in methanol for 5 µL injections.
All SFC experiments were performed on a 1260 Infinity analytical SFC system (Agilent, Waldbronn, Germany) with the following modules: Aurora SFC Fusion A5 module; 1260 Infinity SFC binary pump; 1260 Infinity standard degasse; 1260 Infinity standard autosampler; and 1260 Infinity diode array detector with high pressure SFC flow cell. Unless specifically indicated, the flow rate was conventionally set at 3 mL/min, the temperature of the column compartments at 40 °C and the back pressure of SF-CO2 at 150 bar.
Results and Discussion
Determination of D- and L-Lactic Acid: Lactic acid is an important metabolite produced in several biochemical processes such as anaerobic respiration or fermentation. In mammalian organisms there are two naturally occurring stereoisomers: the L(+)-lactic acid and its counterpart, D(–)-lactic acid, which is present in healthy individuals at around 1% (11). Detection of higher levels of the D enantiomer are indicators of bacterial activity in the intestinal tract or metabolic acidosis (patients with short bowel syndrome, severe gastroenteritis or consequences of jejunoileal surgery).
Figure 4: Chemical structures of the test compounds.
The determination of D- and L-lactic acid in food and clinical samples is therefore of great importance. Currently determination is performed by enzymatic techniques that are time-consuming and are of relatively low quantitative accuracy (12); GC after derivatization (13); and HPLC. The most widely used LC method is based on chiral ligand exchange chromatography with a copper containing mobile phase on Chiralpak MA (14). Its use is therefore limited to UV detection and so mass spectrometry (MS) detection cannot be used.
In this frame, QN AX and QD AX columns allow the enantioselective determination of lactic acid enantiomers using MS-compatible mobile phase systems and offer the possibility of reversing the elution order by switching from one column to the other [Figure 2(b)]. This latter option is not possible with the previously described applications of antibiotic-derived columns, such as Chirobiotic TAG (15).
The mobile phase was composed of an acetonitrile and methanol mixture containing 30 mM formic acid as the additive, and the pH of the mixture was adjusted to pH 4 by the addition of ammonia. This mobile phase provided separations as illustrated in Figure 2. The retention can be adjusted by the additive concentration yielding faster separations when the concentration is increased and longer retention at lower additive concentrations, while enantioselectivities remain nearly constant with such changes.
A preliminary method validation using HPLC UV at 230 nm revealed linearity over two orders of magnitude and allowed LOD (limit of detection) and LOQs (limit of quantifications) below 1 µg and 2 µg respectively, on column (Table 1). For many applications such as in clinical analysis, however, UV detection is not sensitive and selective enough. Owing to its volatile mobile phase constituents, the method is fully compatible with MS detection. Preliminary testing on hyphenation of the above developed enantioselective chromatography with tandem MS (QTrap 4000) showed that this method with QN AX and QD AX respectively is sensitive and selective enough for clinical applications.

Table 1: Performance data for ultra-violet (UV) (230 nm) and electrospray ionization mass spectrometry selected reaction monitoring (ESI-MS SRM) detection using a QN AX column (5 µL injections).
Since lactic acid is a very small molecule, no intensive characteristic fragmentations, except for the transition m/z 89 > 43, could be found. Unfortunately, this transition did not yield an intensive signal. Thus, pseudo-molecular SRM with ion transition of m/z 89 > 89 (in negative ion mode) was selected for quantification, as it is more sensitive than the ion transition which was initially utilized as a qualifier. With these detection parameters a reasonably sensitive method could be defined. The preliminary calibration data and information on sensitivity are summarized in Table 1. It can be seen that the limits of quantitation for MS–MS detection are by a factor of about 100 lower than for UV detection and ranged in the low nanogram level (ca. 10 ng) on-column. The signal was linear over about 20–500 µmol/L. Thus, the method is adequate and can be useful for clinical applications such as diagnosis of diabetic ketoacidosis (16) and other clinical applications in where Dlactic acid may serve as a diagnostic marker.
In addition other α-hydroxy carboxylic acids can be separated (for example, 2-hydroxy butyric acid or glyceric acid), thus, the follow-up analysis of a metabolic change or an enzymatic reaction could be aided by this type of column. This was demonstrated in the recently published article about the determination of D- and L-glyceric acid on a QN AX column (17).
Chromatographic Separation of Circular Plasmid DNA Topoisomers by HPLC: The quinine carbamate CSPs were originally designed for enantiomer separations. However, it was recently discovered that these silica-based stationary phases with quinine carbamate ligands behave like mixedmode reversed-phase/weak anion-exchangers (18) and are potentially useful in other applications as well. Such recently reported applications include among others the separations of hydrophilic peptides with closely related structures including isomers (19). Even more notable, quinine carbamate ligands attached to silica gel have also been demonstrated to be powerful chemoaffinity materials for the chromatographic separation of plasmid topoisomers (20–21).
Plasmid DNA is a popular vector in biotechnology for gene transfer into mammalian cells and is currently being clinically tested for use in human gene therapy. Quality guidelines state that for human use, plasmid DNA should be in its supercoiled isoform, the so-called covalently closed circular (ccc) form. Native ccc form consists of a distribution of individual topological isomers (topoisomers) which differ only in the linking number, that is, by the degree of supercoiling. If a single strand break (nick) occurs in a supercoiled species, the torsional strain is released and an open-circular (oc) form is obtained.

Table 2: The SFC gradient programme. The oc form differs significantly from the ccc form in its hydrodynamic radius and is regarded as an impurity in plasmid DNA (pDNA) samples. Therefore, within the pharmaceutical industry there is a demand for powerful and selective stationary phases, which allow the separation of the two plasmid topologies for quality control. QN AX, along with other quinine-derived CSPs, are able to selectively interact with different supercoiled topoisomers of circular pDNA and allow simultaneous separation of oc form (Figure 3). The peak with the asterisk in Figure 3 represents the oc form which is less strongly bound to the quinine carbamate stationary phase (21). The Gaussian distribution of peaks observed later is the ccc form eluting, with each individual peak constituting a single topoisomer. Adjacent peaks differ in the linking number by a single supercoil. Resolving supercoiling is of significance in various biochemical studies into DNA topology. Furthermore, quinine carbamate stationary phases might also be a useful tool for in-process analysis and quality control in the biotechnological production of pDNA biopharmaceuticals to monitor topoisomer patterns as another process parameter (20).
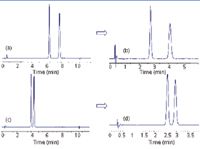
Figure 5: Straightforward method transfer from gradient to isocratic. (a-b) N-Benzoyl-DL-phenylalanine (Compound 12), (c-d) 3-oxo-1-Indancarboxylic acid (Compound 5); Gradient elution: (a) and (c); Modifier % in the isocratic runs: (b) 45%, (d) 30%.
SFC Applications: SFC applications using columns packed with the weak anion-exchanger CSPs have not been investigated until very recently. Although several research groups — mainly in private companies — have undertaken some successful tests on these columns, the efforts focused on specific applications and no systematic investigation was performed. Moreover, the results obtained have not been published due to confidentiality reasons.
In this context, studies of the main parameters to be considered in the SFC mode with anion-exchange selectors have been carried out recently (22-23). The different elements will be discussed in this section.

Figure 6: Dependence of SFC results on percentage of formic acid (FA). Column: QN AX, Modifier: MeOH/NH4OOCH 100/0.35 v/m + FA.
In HPLC, methanol has been demonstrated to be a versatile mobile phase on QN AX and QD AX columns. Owing to its pronounced protic properties, methanol provides efficient salvation of all the ionized species involved in the ion exchange equilibria. Often used as a single solvent in HPLC, the eluotropic strength of methanol can be adjusted by the concentration of the counter-ion (acidic additives) and by the ionic strength (acidic and salt additives).
The same principles can be applied in SFC in terms of the choice for the bulk modifier and the acid salt additives. The investigations performed showed that formic acid (FA) and NH4OOCH added into methanol were leading to remarkable results when used as the modifier of SF-CO2.
From Gradient to Isocratic: The first screening of a series of acidic compounds (Figure 4) was performed by running a SFC gradient programme (Table 2) using a mixture of MeOH/FA/NH4OOCH 100/0.40/0.35 v/v/m as the modifier. Results from the gradient runs allowed easy targeting of the modifier percentage to be used in isocratic mode and compounds to be eluted in a reasonable time window.

Figure 7: Dependence of peak efficiency and resolution degree on flow rate. Compound 11 (N-benzoyl-DL-alanine); (a) peak-2 Column: QN AX; Modifier: 35% (MeOH/FA/NH4OOCH 100/0.40/0.35 v/v/m)
Figure 5 shows the examples of straightforward method transfer from gradient to isocratic conditions. Effects of Mobile Phase Additives: In HPLC, the typical working conditions on QN AX and QD AX columns are with weakly acidic mobile phases (pH 4–7) (6). Under such conditions, the chiral selector is protonated at the quinuclidine ring (Figure 1) and the acidic analytes are dissociated. An ion-exchange mechanism is thus activated between the positively charged chiral selector and the negatively charged analyte molecules.
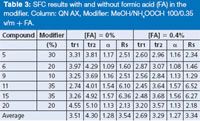
Table 3: SFC results with and without formic acid (FA) in the modifier. Column: QN AX, Modifier: MeOH/NH4OOCH 100/0.35 v/m + FA.
As shown in Table 3, the chiral recognition occurs without external addition of an acidic additive to the methanol/ammonium formate solution. The enantioselectivity was mainly unaffected by the absence or presence of the acidic additive and its concentration [Figure 6(b)]. For most molecules, the addition of FA did not induce notable loss in resolution degree (Table 3). The presence of FA in the mobile phase provided the benefits of reducing the analysis times to a significant extent, especially when exceeding the level of 0.20% [Figure 6(a)]. It is assumed that FA played the role of the counter ion, inducing a competitive effect and favouring the mass transfer kinetics of the chromatographic process.
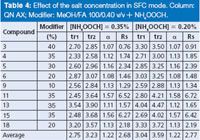
Table 4: Effect of the salt concentration in SFC mode. Column: QN AX; Modifier: MeOH/FA 100/0.40 v/v + NH4OOCH.
The ion-exchange chromatographic process is dependent not merely on the ionization state of the chiral selector and the analyte molecules, but also on the ionic strength of the mobile phase. Addition of a certain amount of a suitable salt into the mobile phase can efficiently modulate the ionic strength of the mobile phase and regulate the adsorptiondesorption process between the analyte and the chiral selector. In practice, it is preferable to choose a salt of high solubility in the modifier or the mobile phase, high volatility for the potential LC–MS hyphenation and high UV transparency to ensure good UV detection of the analytes. NH4OOCH meets all these requirements.
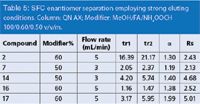
Table 5: SFC enantiomer separation employing strong eluting conditions. Column: QN AX; Modifier: MeOH/FA/NH4OOCH 100/0.60/0.50 v/v/m.
NH4OOCH was added to the methanolic modifier at two different concentrations: 0.35% and 0.20%. As shown in Table 4, the enantioselectivity was unaffected by the concentration of NH4OOCH. The higher concentration of NH4OOCH led to reduced retention times at about 10% with minor diminution in resolution degree of the enantiomers. The concentration of 0.35% NH4OOCH in MeOH seems to be a good choice for generic method development. Caution would be needed if significantly higher salt concentrations are required, because of the unknown solubility of the salt in SF-CO2.
It is worth noting that a binary mixture of methanol/FA with no salt is not a stable system as a result of the rapid esterification reaction. It would lead to an increasing sample retention over time for a given analysis. The presence of the salt NH4OOCH in the modifier plays the role of the "stabilizer" of the system and is essential for reproducible chromatographic results.

Table 6: Comparison between QN-AX and QD-AX columns in SFC. Modifier: MeOH/FA/NH4OOCH (100/0.40/0.35 v/v/m).
Elution of Strongly Retained Compounds in SFC: From the experimental data the mixture of MeOH/FA/NH4OOCH at the proportion of 100/0.40/0.35 v/v/m represents a reasonable compromise in terms of elutropic strength and can be used as a starting point for SFC method development. However, a few of the acidic compounds under investigation showed stronger retention on the columns and could not be (completely) eluted with such a methanolic modifier in the gradient as described before.
In this scenario, efficient elution could be achieved by using a high percentage of the modifier (50–60%) containing slightly higher concentrations of the acid and salt additives and, if necessary, increasing the flow rate. Some chromatographic results obtained under the enhanced eluting conditions are presented in Table 5.
It is worth mentioning that the chiral stationary phases show relatively slow mass transfer kinetics. This drastic variation of the resultant resolution degree in dependence on the flow rate is shown in Figure 5. Thus, an effective means for improving the enantiomer separation is to reduce the flow rate.
Elution Order and Complementarity: As described in Figure 1, QN and QD are diastereomers. Chromatographically, the two CSPs behave as "pseudoenantiomers" because the stereoselectivity is under the control of C8 and C9 which have the opposite configurations.
Consequently, the elution order (EO) of the enantiomers is reversed on these two columns, as described previously for the HPLC mode (Figure 2). In the current SFC investigation, such a phenomenon was monitored by injecting one of the pure enantiomers which were available in our laboratory (Table 6). Coincidently, all the four D enantiomers examined were first eluted on QN AX and secondly eluted on QD AX. The feasibility of choosing the column in terms of elution order is undoubtedly of value especially for applications in which one enantiomer is present at a very low percentage (< 1%). Elution of the trace enantiomer in front of the major enantiomer is usually beneficial.
Conclusions
Quinine- and quinidine-derived anion exchangers are versatile in enantiomer resolution of acidic compounds by LC and SFC. While their use in HPLC is well established, their application in SFC is still a working topic that needs to be developed.
The major factors influencing the chiral separation in SFC include the acidic additive, the salt concentration and the flow rate. The mixture of MeOH/FA/NH4OOCH 100/0.40/0.35 v/v/m seems to be a suitable modifier for the first trials of method development in SFC. It was also observed that the enantiomer separations by SFC are minorly affected by the temperature in the range of 20 °C and 40 °C. Lower modifier percentages usually lead to longer retention times but have no major effect on enantioselectivity and resolution degree. The effect of other polar organic modifiers (such as ethanol, 2-propanol and acetonitrile) as alternatives to methanol was not investigated because of their poor solubility to the salt additives and the resultant potential inconvenience in the balance of eluotropic strength of the mobile phase.
These chiral columns exhibit interesting selectivities in various separations beyond enantiomer resolution. The remarkable topoisomer selectivity of quinine carbamate phases was illustrated herein as an example. The applications of QN AX and QD AX columns in non-chiral applications has not been fully explored and deserves further investigation.
Pilar Franco received her PhD degree in Pharmacy from the University of Barcelona, Spain. After two years postdoctoral work in the University of Vienna, Austria, under the supervision of Professor Wolfgang Lindner, she joined Chiral Technologies Europe, Strasbourg, France. She is now Manager of Technical Operations in the same company. Her main interests include the applications of chiral selectors for the resolution of enantiomers at analytical and preparative scale.
Tong Zhang received her PhD degree in Organic Chemistry from the University of Bordeaux I, Bordeaux, France. After three years postdoctoral work at Ciba Geigy, Basel, Switzerland, under the supervision of Dr Eric Francotte, she joined Chiral Technologies Europe, Strasbourg, France. She is now the R&D Manager in the same company. Her main interests include development and applications of chiral stationary phases for resolution of stereoisomers by chromatography.
Andrea Gargano has a Master in Pharmaceutical Chemistry and Technology from the University of Pavia, Italy. Currently he is a PhD student in analytical chemistry at the University of Vienna, Austria. His expertise includes liquid chromatography, HILIC, mixed mode, chiral chromatography on silica-based stationary phases and bioanalytics on organic monoliths.
Marek Mahut received his PhD in 2010 from the Department of Analytical Chemistry, University of Vienna (Austria). His PhD work focused on separation media and methods for plasmid DNA analysis. He then occupied a position in analytical development with the Austrian pharmaceutical company Sanochemia Pharmazeutika. He recently joined the technical R&D of Novartis Pharma in Basel, Switzerland.
Michael Lämmerhofer earned his PhD in Pharmaceutical Chemistry at the University of Graz, Austria. He was coworker of Professor W. Lindner (University of Vienna, Austria) until 2011 and from 1999 to 2000 he was post-doc at the Department of Chemistry of the University of California, Berkeley, USA, with Prof Frantisek Svec. He is now Professor for Pharmaceutical (Bio)Analysis at the University of Täbingen, Germany. His research interests include the development of functionalized separation materials, monoliths, nanoparticles, metabolomics and plasmid DNA analysis.
Wolfgang Lindner has occupied the prestigious chair of Analytical Chemistry at the University of Vienna, Austria, since 1996. His pioneering and ground-breaking work in molecular recognition and on stereo-selective techniques has ensured that he is recognized as the founder of such technologies on synthesis and mechanistic understanding of anion, cation and zwitterion exchange chiral stationary phases for chromatographic separations.
References
(1) A. Mandl, L. Nicoletti, M. Lämmerhofer and W. Lindner, J. Chromatogr. A. 858 (1), 1–11 (1999).
(2) M. Lämmerhofer and W. Lindner, J. Chromatogr. A. 741 (1), 33–48 (1996).
(3) M. Lämmerhofer, J. Chromatogr. A. 1217(6), 814–856 (2010).
(4) V. Piette, M. Lämmerhofer, K. Bischoff and W. Lindner, Chirality, 9(2), 157–161 (1997).
(5) M. Lämmerhofer, D. Hebenstreit, E. Gavioli, W. Lindner, A. Mucha, P. Kafarski and P. Wieczorek, Tetrahedron Asymm. 14 (17), 2557–2565 (2003).
(6) M. Lämmerhofer, P. Imming and W. Lindner, Chromatographia supplement 60(1), S13–17 (2004).
(7) M. Lämmerhofer, O. Gyllenhaal and W. Lindner, J. Pharm. Biochem. Anal. 35(2), 259–266 (2004).
(8) M. Lämmerhofer, N.M. Maier and W. Lindner, American Laboratory. 30, 71–78 (1998).
(9) W.R. Oberleitner, N.M. Maier and W. Lindner, J. Chromatogr. A. 960(1–2), 97–108 (2002).
(10) E. Zarbl, M. Lämmerhofer, F. Hammerschmidt, F. Wuggenig, M Hanbauer, N.M. Maier, L. Sajovic and W. Lindner, Analytica Chimica Acta. 404(2), 169–177 (2000).
(11) J.B. Ewaschuk, G.A. Zello, J.M. Naylor and D.R. Brocks, J. Chromatogr. B. 781(1–2), 39–56 (2002).
(12) http://www.biocontrolsys.com/products/EnzyPlusDlactic.html
(13) F. Betschinger, J. Libman and A. Shanzer, J. Chromatogr. A. 46(1), 53–62 (1996).
(14) O.O. Omole, D.R. Brocks, G. Nappert, J.M. Naylor and G. A. Zello, J. Chromatogr. B. 727(1–2), 23–29 (1999).
(15) D. Norton, B. Crow, M. Bishop, K. Kovalcik, J. George and J.A. Bralley, J. Chromatogr. B. 850(1–2), 190–198 (2007).
(16) J. Lu, C.A. Zello, E. Randell, K. Adeli, J. Krahn and Q.H. Meng, Clinica Chimica Acta. 412(3–4), 286–291 (2011).
(17) H. Wulf, M. Perzhorn, G. Sievers, F. Scholz and T. Bornscheuer, J. Mol. Catalysis B: Enzymatic. 74(1–2), 144–150 (2012).
(18) M. Lämmerhofer, M. Richter, J. Wu, R. Nogueira, W. Bicker and W. Lindner, J. Sep. Sci. 31(14), 2572–2588 (2008).
(19) S. Schiesel, M. Lämmerhofer, A. Leitner and W. Lindner, J. Chromatogr. A. 1259, 111–120 (2012).
(20) M. Mahut, E. Haller, P. Ghazidezfuli, M. Leitner, A. Ebner, P. Hinterdorfer, W. Lindner and M. Lämmerhofer, Angew. Chem. Int. Ed. 51(1), 267–270 (2012).
(21) M. Mahut, W. Lindner and M. Lämmerhofer, J. Am. Chem. Soc. 134 (2), 859–862 (2012).
(22) T. Zhang, N. Nguyen, P. Franco and M. Vollmer, Agilent application note (2011): (http://www.chem.agilent.com/Library/applications/5990-9459EN.pdf)
(23) R. Pell and W. Lindner, J. Chromaogr. A. 1045 (1–2), 175–182 (2012).

Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.








