Pragmatic Rules for GC Column Selection
An excerpt from LCGC's e-learning tutorial on GC column selection at CHROMacademy.com
Page 126
An excerpt from LCGC's e-learning tutorial on GC column selection at CHROMacademy.com
In terms of selecting an appropriate stationary phase, there are four primary analyte or stationary-phase interactions that need to be considered.
Dispersive interactions (<<1 kJ/mol) are lower energy (van der Waals) forces between nonpolar moieties of the analyte molecule, that is, C–H bonds and so on. These will be in play when using any silica-based stationary phase because the majority of the phase polymeric backbone (polydimethylsiloxane [PDMS]) is nonpolar in nature.
Dipole–dipole and dipole–induced dipole interactions (3 and 1 kJ/mol, respectively) are in play whenever unsaturated, aromatic, or more-polar functional groups (that is, C–Cl or C–N bonds) are present in the stationary phase or analyte molecule. Stationary phases containing phenyl, cyano, or trifluoro functional groups are more polar than PDMS, and the more of these functional groups there are, the greater their influence is on the separation. The increase in retention of aromatic compounds and the relative decrease in retention of aliphatic analytes when moving from a 5% phenylmethyl PDMS phase to a 50% phenylmethyl PDMS phase exemplifies this.
Hydrogen bonding interactions (19 kJ/mol) are the strongest intermolecular forces in capillary gas chromatography (GC) and occur whenever the stationary phase contains cyano, trifluoro, or (especially) hydroxyl functional groups. This type of force is in play when analyzing alcohols using a polyethylene glycol or "wax" type phase.
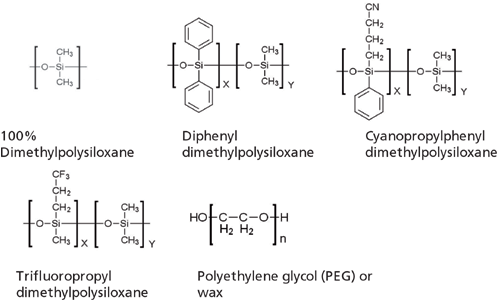
Figure 1: The five most common GC column stationary-phase chemistries.
Pragmatic phase-selection rules can be summarized as follows:
- Use the principles of "like dissolves like" wherever possible and match the polarity of the analyte to the polarity of the stationary phase.
- Remember that there are only really five "chemistries" that we need to consider (see Figure 1). To increase retention or alter selectivity based on a particular interaction, increase the amount of the functional group within the phase (that is, move from a 14% to a 35% cyanopropyl phase).
- Use the least-polar phase possible because more-polar phases bleed more (it's inherent in the chemistry).
- A 5% phenyl column can be used to screen unknown samples - analyte retention and selectivity can then be assessed and a more appropriate phase chosen if necessary.
- A 5% phenyl, 50% phenyl, 14% cyanopropyl, and a wax (polyethylene glycol [PEG]) column cover the widest range of possible interactions (stationary-phase polarities) in the fewest number of columns.
How does one select the physical column dimensions of length (L), internal diameter, and film thickness (df)?
Column length affects the separation efficiency and therefore the resolution. Doubling column length doubles efficiency (number of theoretical plates [N]), doubles analysis time in isothermal separations (1.5–1.75× increase if using gradient temperature programming), doubles column cost, and increases resolution by a factor of 1.4. Increasing column length is the worst way to improve the resolution of a separation; however, when you have a sample with many components (hundreds) you sometimes need a long column. Select column length according to the number of species that need to be separated in the sample. For two components use a 10-m column, and for hundreds of components use a 60-m or 120-m column.
Column internal diameter affects retention and efficiency. Halve the column internal diameter, double the efficiency, and increase resolution by a factor of 1.4. This will double retention time only for isothermal separations and only if the film thickness in not altered. The phase ratio (β) is equal to the column radius (mm) divided by 2× the film thickness (µm). Keep the phase ratio constant between columns and the retention time will be approximately constant. Use β < 100 for highly volatile analytes and β > 400 for high-molecular-weight analytes or for trace analysis. Use smaller internal diameter columns when the separation is dependent on the stationary phase selectivity (analytes chemically very similar) or when multiple components need to be separated in shorter time-frames. Note that the column capacity will decrease as the column internal diameter is reduced.
Film thickness affects retention of analyte species, interaction with the silica tubing, phase bleed, and column capacity. Doubling the film thickness doubles retention time for isothermal analysis and increases retention by a factor of around 1.5 for temperature-programmed analysis. Doubling film thickness increases elution temperature by around 20 °C. Use thin films (0.1–0.25 µm) for trace analysis or when analytes are relatively involatile. Use thicker films (1–5 µm) when dealing with volatile analytes, analytes at high concentration, or when peak shape is poor. Note that increasing film thickness may compromise resolution for later-eluted analytes (retention factor > 5) and that phase bleed and column capacity increase with increasing film thickness.

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











