Fluorescence-Detection Size-Exclusion Chromatography — An Analytical Technique with Multiple Applications
LCGC Europe
Fluorescence-detection size-exclusion chromatography and its implications in protein characterization, specifically the development and characterization of biologics, is reviewed.
Pages 118-125
In this instalment, we discuss fluorescence-detection size-exclusion chromatography (FSEC) technology and its implications in protein characterization, specifically the development and characterization of biologics.
There are various techniques available for the detection and separation of proteins. Fluorescence-mediated detection is considered to be highly sensitive for cell and molecular biology analytical methods. For applications involving enzyme-linked assays, DNA sequencing, confocal microscopy, and cell biology, fluorescence detection approaches rely on the self-luminous properties of fluorescent molecules (1). There are several fluorescent tags available including Cy3, Cy3.5, Cy5, fluorescein, TA (2,4-chloro-[6-(4-(N,N'-diethylaminophenyl)-1,3,5-triazine]), as well as green fluorescent protein (GFP) and enhanced green fluorescent protein (EGFP) variants such as enhanced blue fluorescent protein (EBFP), enhanced cyan fluorescent protein (ECFP), and enhanced yellow fluorescent protein (EYFP). Among these, GFP and EGFP are the most commonly used fluorescent tags because they are resistant to heat, alkaline pH, detergents, photobleaching, chaotropic salts, organic salts, and several proteases (2). On the other hand, disadvantages of the GFP tag include slow post-translational chromophore formation and difficulty in distinguishing GFP from background fluorescence when GFP is not densely localized or highly expressed (3). Although there are many other fluorescent proteins available commercially, GFP and its derivatives are still commonly used as a fusion tag for many proteins in living cells and many in vivo experiments because of convenience of noninvasive monitoring. In addition, fluorescent proteins are advantageous as fusion tags for recombinant protein expression in vitro. Fluorescence-detection size-exclusion chromatography (FSEC) is mostly used for precrystallization screening and membrane protein expression studies. In general, the protein of interest is tagged with GFP and expressed in an appropriate host. Protein expression level is analyzed by size-exclusion chromatography (SEC) linked to fluorescence detection (Figure 1) using a high performance liquid chromatography (HPLC) instrument. In this instalment, we discuss the FSEC technique and its implications in protein characterization, specifically the development and characterization of biologics.
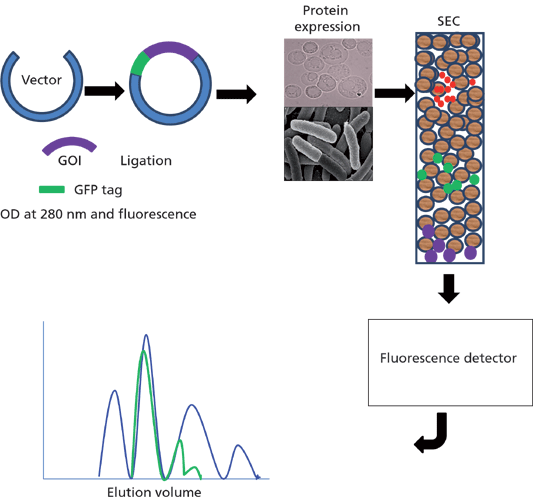
Figure 1: Fluorescence size-exclusion chromatography (FSEC).
Precrystallization Screening
FSEC is well-suited for precrystallization screening. In general, membrane proteins are usually extracted with detergents and are highly flexible and unstable in nature (4). The study of membrane proteins is also difficult because of their intrinsic surface hydrophobicity. Receptors like G protein-coupled receptors (GPCRs), ion channel proteins, and other integral membrane proteins are difficult to over-express in functional form. Loss of function could also occur because of the aggregation of proteins before purification and crystallization. Various protein solubilization conditions and techniques are used to address such protein aggregation. Most often, detergents and additives such as lipids, ligands, and fusion protein approaches are used. These lengthy and tedious processes are quite promising, but nonetheless do not ensure success (5). Precrystallization screening using FSEC can substantially improve the probability of successful crystallization (6). Techniques like thermal shift analysis of protein to understand protein folding, stability, and structural complexity could easily be coupled with FSEC, which is known as FSEC-TS (7).
Recombinant Protein Expression
GFP and red fluorescent proteins (RFP) are often used to visualize tagged proteins in cloning, expression, chromatography purification, crystallization, and protease-cleavage experiments. Mammalian vectors such as pEGFP, pYFP, pCFP, or pBFP use GFP variants as fusion protein partners; albeit the lack of a binding ligand for GFP proteins restricts the usefulness of such expression systems (8). Other than fluorescence tags, polyhistidine, maltose binding domain, and glutathione S-transferase are extensively used as fusion protein tags to ease protein purification in recombinant systems. Similar to other tags, GFP variants solubilize easily under physiological conditions and this property helps in enhancing solubility of the protein of interest. Membrane proteins, G protein-couple receptors, kinases, and ion channel proteins have been overexpressed as fluorescently tagged fusion proteins in E. coli, insect cell lines (for example, Sf9, Hi5, Sf21), and mammalian cells (for example, HEK 293 cells). Overexpressed protein purified from such platforms was used in FSEC studies for rapid testing of expressibility, stability, and monodispersity.
Protein expression screening methods are important tools in proteomics for finding high expressing protein targets. Expressed proteins can be labelled either as fusions with fluorescent protein (such as GFP) or through translational incorporation of a fluorescent amino acid derivative like BODIPY-FL-lysine. Fluorescence-based detection of such labelled proteins is routinely carried out; more importantly, analysis of protein expression through FSEC helps in rapid identification of the optimal target (9). FSEC can also be used in studying the expression of monoclonal antibodies and antibody fragments by tagging with a fluorescent tag. Antibody glycan variants play a major part in monoclonal antibody development and help in protein stability. Tagging the glycans of monoclonal antibodies or with fluorescent labels such as 2-aminobenzamide (2-AB) might allow the glycoprotein to be detected at very low concentration (femtomoles). Other interesting applications using FSEC include protein aggregation, protein stability, and protein structure function analysis. Human erythropoietin aggregates from formulated product have been analyzed using a size-exclusion HPLC method with fluorescence detection (10). Size-exclusion HPLC methods coupled with intrinsic fluorescence detection have been developed for evaluating the stability and degradation profiles of interferon alpha-2 drug substances and the same approach has been applied for analyzing a number of biotherapeutic proteins (11) in a high-throughput manner. Multicolour fluorescence size-exclusion chromatography has been used to analyze multimeric protein complexes by tagging different subunits with separate fluorescent tags. This technique aids researchers to track the functionality of individual subunits through the expression, solubilization, and purification steps and finally to analyze membrane multisubunit assemblies.
Gene Knockdown
Genetic manipulation studies in mammalian and other higher eukaryotic cell lines is an important research and development area to understand the underlying biology of biochemical pathways. In recent years, technological advancement in gene knock-in and knockout has allowed researchers to study the functionality of any gene of interest. Various technologies like homologous recombination mediated genetic manipulation, microRNA-based gene knockout, small interfering RNA-based methods are used to achieve specific gene knockout cell lines. Fluorescence-based detection technologies like FSEC play an important role in identifying and selecting cells after targeted gene knockdown. Small interfering RNA (siRNA) has emerged recently as a powerful method for gene silencing or knockdown of gene expression. In this technique, double-stranded RNA induces the degradation of message sequences ultimately resulting in depletion of the encoded protein (12). There are hairpin siRNA vectors containing fluorescent tags under the control of a strong promoter that is transfected into the mammalian cells and silencing the expression of the targeted gene. Retroviral vectors that express small hairpin RNA (shRNA) for gene knockdown are available commercially with constitutive U6 promoter with fluorescent protein reporter. Another important tool to suppress or stop the expression of target genes is microRNA-based gene knockdown. Lentiviral vector for gene knockdown allows for the direct cloning of shRNA oligos using alpha-complementation, monitoring of induction of RNA interference with fluorescent protein and the percentage of gene knockdown can be studied using FSEC (13). Comparison of protein products from cells with gene knockdown and untransfected control cells will provide evidence for gene knockdown.
Gene Expression or Gene Transfer
A crucial aspect of gene expression studies is selection through a better reporter gene. The use of GFP and other fluorescent proteins in gene expression vectors serve an important step in such studies. Gene transfer in its most useful sense must not only include monitoring the successful transfer of the gene of interest, but also the establishment of the correct and predictable pattern of transgene expression (14). Tagging of single transcripts with two fluorescent markers can be used to study many aspects of gene expression. The LacZ gene, encoding β-galactosidase from E.coli, was first used for single-cell gene expression analysis in Caenorhabditis elegans and other cells in 1990. LacZ was the reporter gene of choice until the introduction of GFP in 1994. The primary advantage of GFP over LacZ is the ability to visualize reporter gene expression in live animals rather than in fixed preparations. Therefore, GFP can be more easily used for a variety of applications with a high success rate, which includes gene expression pattern analysis; dissection of cis-regulatory sequences; protein localization in specific cells or tissues; visualization of cellular anatomy (such as neuroanatomy); cell identification; and visualization of cellular and physiological processes. For the reasons cited above, GFP reporter transgenes have become the primary tool for gene expression analysis in C. elegans as well as in other model organisms (15). Expression of the fusion constructs containing fluorescent tags in transgenic worms allows the determination of the developmental stage, tissue, and in some cases, the cells where the gene of interest is expressed. Figure 2 illustrates a possible strategy for studying gene expression. FSEC plays a role in screening a large number of clones for future studies and also checks the movement of the fluorescent-labelled gene of interest efficiently (16).
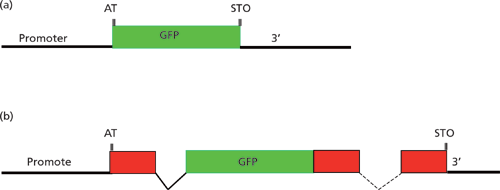
Figure 2: Architecture of reporter gene constructs: (a) Transcriptional reporter, (b) translational reporter. Noncoding DNA sequences are indicated by black lines. Exon sequences are represented as red boxes. GFP is shown in green. Adapted from reference 15.
Protein–Protein Interaction
Knowing protein structure and function is essential in understanding cellular localization, protein–protein interactions, and the biochemical pathways it may be involved in. Protein interactions are fundamental mechanisms for biological activity and play pivotal roles in carrying out many cellular functions that serve as the basis of signal transduction pathways in cells. FSEC can serve as a replacement to understand protein–protein interactions compared to fluorescence-lifetime imaging microscopy (FLIM) and fluorescence resonance energy transfer (FRET) techniques. Known proteins can be tagged with fluorescent protein and transfected into appropriate host cells such as E.coli, insect cells, mammalian cell lines, and animal models. After cell lysis or tissue lysis (in the case of animal models), the crude sample or fractions can be analyzed using FSEC to study the interacting partner. FSEC can also help in studying heteromeric protein interactions (Figure 3) for finding unknown targets. In cancer biology, this technique is very useful in studying protein-DNA interactions by tagging a fluorescent tag to the protein of interest. Elucidation of protein interaction networks also contributes greatly to the analysis of signal transduction pathways. Recent developments in this field of research have elucidated unique biochemical pathway networks comprised of signalling pathways and thereby identifying novel protein complexes as targets for specific diseases (17).
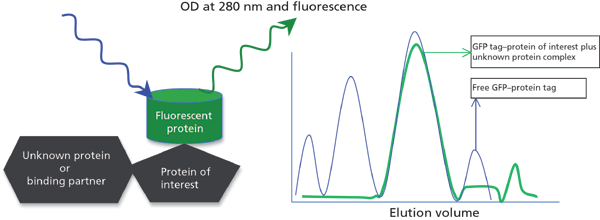
Figure 3: Protein-protein interaction.
Intracellular Signalling
Subcellular fractionation and protein enrichment are two very important approaches in drug discovery process. The isolation of less abundant proteins from subcellular fractions allows more efficient identification and characterization studies of proteins of interest, when tagged with fluorescence tags. Live cell imaging of fluorescent protein tags allows the investigator to examine protein distribution in living unadulterated cells, thus avoiding potential artifacts because of cell fixation and permeabilization. GFPs and RFPs have been used to observe real time movements of many signalling proteins including transcription factors, kinases, and other signalling proteins involved in biochemical pathways (18). FSEC can be used to check for the presence of a protein of interest fused with GFP by isolating particular cellular organelles, which is a huge contribution in understanding critical coordination of various organelles.
In Vivo Biology
Animal model studies are more complex than cellular level understanding of bioprocesses. Fluorescent protein-based studies allow researchers to visualize, in real time, important aspects of cancer progression in living animals, including tumour cell mobility, invasion, metastasis, and angiogenesis. After expression, particular organs or cells can be isolated and studied using FSEC, making this a promising technology to study biological processes. As a tool, FSEC can easily be adopted to study biological events like cancer gene expression, ion fluxes, protein and organelle trafficking, chromosome dynamics, and numerous other processes that can presently be examined only through in vitro studies (19). It is possible to transduce GFP tagged molecules in primary (that is, growing) tumour cells in mouse models through simple injection of GFP retroviral supernatants, subsequently lymphatic, liver, and other metastasis are examined. Follow-up analysis of GFP expressing tissues and cell types using FSEC helps in monitoring tumour progression and plausible target molecules to restrict the disease progression (20). The availability of GFP variants with nonoverlapping emission spectra (CFP, YFP) has created the possibility for multicolour-labelling of cells in vivo. Various classes of neurons can be labelled efficiently by triple colour combinations (CFP, YFP, and Discosoma sp. red fluorescent protein [DsRed]). Sophisticated detectors with appropriate filter sets have been integrated in various instrument platforms to detect and analyze variants of CFP and YFP.
Plant Cell Culture
Transgene expression using Agrobacterium tumefacienes can be confirmed by FSEC. GFP, modified versions of GFP, and other fluorescent protein tags are convenient and sensitive reporters for the visualization of gene regulation, signal transduction, protein–protein interactions, and subcellular localization of chimeric proteins in living plant cells like maize, onion, Arabidopsis, and transgenic tobacco plants (21). The fluorescent tag can be inserted into the plant expression vector and after expression, the plant cells can be analyzed using FSEC. The applications of this approach can be utilized in production of therapeutic or diagnostic monoclonal antibodies, vaccines, and other biopharmaceutical proteins (22). A large number of researchers have focused on expression of protein-based drugs in plant systems and a few of them are in different stages of clinical trials. Therapeutic protein product expression could be screened using FSEC.
Advantages and Disadvantages
Advantages of using FSEC include its small sample requirement and ability to perform analyses in a high-throughput format. The analysis itself is rapid, reliable, and economical and can reduce false positive results by analyzing the background fluorescence. This technique can also be used for monitoring protein expression level, identifying novel proteins, and studying cellular signalling pathways in cells or animal models. The protein sample could be collected from any source such as bacteria, mammalian cells, and tissue. FSEC can also be coupled with thermal shift analysis by incubating the protein sample at temperature gradient to understand melting points, molecular weight, and the presence of aggregates. Multiple detection schemes such as UV and fluorescence can be combined for purity profiling in SEC. The major drawback of this technique, however, is that if the sample is crude, the sample may sometimes be eluted with the aggregates in SEC and result in a false-positive result.
Summary
This review focuses on the use of FSEC as an analytical tool in a variety of applications pertaining to discovery, development, and commercialization of protein therapeutics. Recent technological advancements in the field of fluorescence markers and corresponding detection methodologies made it possible to identify novel therapeutic targets. Moreover, characterization of biotherapeutic candidates such as monoclonal antibodies, neutralizing antibodies, and bispecific antibodies through advanced technologies like FSEC facilitated successful progression from early discovery and development stages to marketing approval. FSEC is an excellent analytical technique for qualitative and quantitative analysis of potential drug molecules. However, the method is not yet suitable for large-scale separations of biotherapeutics at the manufacturing scale. Future research will focus on developing a robust platform to analyze samples from different cells and animal models using FSEC.
Sunit Maity has been associated with Theramyt Novobiologics since its inception and currently heads the product development team.
Vivek Halan has a postgraduate degree in biotechnology from Bharathidasan University in Trichy, Tamilnadu. Currently, Vivek heads the downstream department at Theramyt Novobiologics.
Ira S. Krull is a Professor Emeritus with the Department of Chemistry and Chemical Biology at Northeastern University in Boston, Massachusetts, USA, and a member of LCGC's editorial advisory board.
Anurag S. Rathore is a professor in the Department of Chemical Engineering at the Indian Institute of Technology in Delhi, India.
References
(1) M. Whitaker, BioEssays22, 180–187 (2000).
(2) M.A. Ehrmann, C.H. Scheyhing, and R.F. Vogel, Lett. Appl. Microbiol.32, 230–234 (2001).
(3) M. Zimmer, Chem. Rev.102, 759–81 (2002).
(4) E.P. Carpenter, K. Beis, A.D. Cameron, and S. Iwata, Curr. Opin. Struct. Biol. 18, 581–586 (2008).
(5) D. Parcej, R. Guntrum, S. Schmidt, A. Hinz, and R. Tampe, Plos One8, e67112 (2013).
(6) T. Kawate and E. Gouaux, Structure14, 673–81 (2006).
(7) M. Hattori, R.E. Hibbs, and E. Gouaux, Structure20, 1293–1299 (2012).
(8) W.W. Su, Microb. Cell Fact4, 12 (2005).
(9) M.A. Coleman, V.H. Lao, B.W. Segelke, and P.T. Beernink, J. Proteome. Res.3, 1024–32 (2004).
(10) S.R. Gunturi, I. Ghobrial, and B. Sharma, Pharm. Biomed. Anal.43, 213–221 (2007).
(11) A. Diress, B. Lorbetskie, L. Larocque, X. Li, M. Alteen, R. Isbrucker, and M. Girard, J. Chromatogr.1217, 3297–3306 (2010).
(12) S. Kojima, D. Vignjevic, and G.G. Borisy, BioTechniques36, 74–9 (2004).
(13) M.F. Alexeyev, R. Fayzulin, I.N. Shokolenko, and V. Pastukh, Mol. Biol. Rep.37, 1987–91 (2010).
(14) S. Welsh and S.A. Kay, Curr. Opin. Biotechnol.8, 617–622 (1997).
(15) T. Boulin, J.F. Etchberger, O. Hobert, and H. Hughes, WormBook: The Online Review of Caenorhabditis elegans Biology, Nucl. Acids Res. 35 (suppl 1) (2007): D472–D475.
(16) S. Hocine, P. Raymond, D. Zenklusen, J.A. Chao, and R.H. Singer, Nat. Methods10, 119 (2013).
(17) V.S. Rao, K. Srinivas, G.N. Sujini, and G.N.S. Kumar, Int. J. Proteomics2014, 1–12 (2014).
(18) J.M. Tavare, L.M. Fletcher, and G.I. Welsh, J. Endocrinol.170, 297–306 (2001).
(19) R.M. Hoffman, Acta Histochem.106, 77–87 (2004).
(20) S. Hasegawa, M. Yang, T. Chishima, Y. Miyagi, H. Shimada, A.R. Moossa, and R.M. Hoffman, Cancer Gene Ther.7(10), 1336–1340 (2000).
(21) W. Chiu, Y. Niwa, W. Zeng, T. Hirano, H. Kobayashi, and J. Sheen, Curr. Biol.6, 325–330 (1996).
(22) K. Ko, R. Brodzik, and Z. Steplewski, Curr. Top Microbiol. Immunol.332, 55–78 (2009).

Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
Using GC-MS to Measure Improvement Efforts to TNT-Contaminated Soil
April 29th 2025Researchers developing a plant microbial consortium that can repair in-situ high concentration TNT (1434 mg/kg) contaminated soil, as well as overcome the limitations of previous studies that only focused on simulated pollution, used untargeted metabolone gas chromatography-mass spectrometry (GC-MS) to measure their success.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.
Potential Obstacles in Chromatographic Analyses Distinguishing Marijuana from Hemp
April 28th 2025LCGC International's April series for National Cannabis Awareness Month concludes with a discussion with Walter B. Wilson from the National Institute of Standard and Technology’s (NIST’s) Chemical Sciences Division regarding recent research his team conducted investigating chromatographic interferences that can potentially inflate the levels of Δ9-THC in Cannabis sativa plant samples, and possible solutions to avoid this problem.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












