Analysis of Complex Pharmaceuticals by Ultrahigh-Pressure Liquid Chromatography: Case Studies and Quality Control Implications
LCGC Europe
Experimental results and method validation data from two analytical case studies of complex small?molecule pharmaceuticals illustrates the utility and advantages of UHPLC in high-resolution separations.
Volume 28 Number 2
Pages 88-97
In this article, we present experimental results and method validation data from two analytical case studies of complex small-molecule pharmaceuticals to illustrate the utility and potential advantages of ultrahigh-pressure liquid chromatography (UHPLC) in high-resolution separations, particularly for quality control purposes. The focus is on the use of UHPLC in potency assays and purity analysis of complex drug substances and drug products using UV detection, where resolution of all analytes is required. Finally, we discuss some potential technical issues and mitigation strategies for conducting these high-resolution chromatographic analyses in a regulated environment.
Ultrahigh-pressure liquid chromatography (UHPLC) instrumentation is rapidly emerging as the standard modern high performance liquid chromatography (HPLC) platform (1–7). Today, most major manufacturers offer some type of UHPLC system with upper pressure limits of 15,000–22,000 psi (1000–1500 bar) (2). While features and configurations are diverse, all UHPLC systems are designed to have lower system dispersion and gradient dwell volumes and increased precision performance compared to conventional HPLC systems (2,8,9). The benefits of UHPLC have been well reviewed (8–12). Most practitioners are attracted to this technology because of its capability for faster analysis with good resolution and rapid method development. Another significant benefit of UHPLC is its ability in high-resolution chromatography using long columns packed with small particles. While this aspect is predictable from chromatographic principles, a literature search yielded few references on this topic in pharmaceutical analysis (7–9,10–17), particularly in quality control (QC) applications

(PHOTO CREDIT: FOTOSEARCH/GETTY IMAGES)
In this article, we describe the results of two case studies on the analyses of complex pharmaceuticals (drug molecules with multiple stereogenic centres and drug products from multiple active pharmaceutical ingredients [APIs]). In each case, we describe the separation challenges, method development or improvement strategies, final operating UHPLC conditions, and method performance and validation data. These examples illustrate the practical implementation of UHPLC with acceptable method performance for complex pharmaceuticals. Finally, we discuss some potential issues and implementation hurdles of UHPLC in QC applications as well as their mitigation strategies.
Experimental
Chemical and Reagents: ACS-grade reagents (ammonium hydroxide, phosphoric acid, formic acid, and potassium monobasic phosphate) were obtained from J.T. Baker. Ammonium formate (99.999% purity) and formic acid (99%+) were obtained from Aldrich Chemicals. HPLC-grade solvents (acetonitrile) were obtained from EMD Chemicals. Purified HPLC-grade water (>18.2 MÎ) was obtained from PureLab Ultra water purification systems from Elga or from a Milli-Q water purification system from Millipore. New chemical entities and their related substances used in the case studies were synthesized at Genentech or Gilead Sciences.
Instrumentation and Columns: The systems used in the first case study were Agilent 1290 UHPLC systems equipped with binary pumps, autosamplers, column ovens, and photodiode array detectors and similarly equipped conventional Agilent 1200 HPLC systems. Waters Acquity UPLC systems equipped with quaternary pumps (H-Class), autosamplers, column ovens, and photodiode-array detectors (0.5-µL cell volume, 10-mm flow cell) were used in the second case study. Details on these systems including specifications of all corresponding modules are available from the manufacturers' respective websites. The column and operating conditions used in each study are identified in the captions of the figures. An HPLC method development software package (Fusion AE) obtained from S-Matrix Corporation was used for method development and optimization in the second case study.
The HPLC columns used included 150 mm × 2.1 mm, 1.7-µm dp Waters Acquity BEH C18 and BEH Shield RP18 columns, 150 mm × 4.6 mm, 3.0-µm dp MacMod Analytical ACE-3-C18 columns, and 100 or 150 mm × 3.0 mm, 2.0-µm dp ACE Excel 2 C18 columns, also from MacMod Analytical.
Results and Discussions
Case Study 1: Achiral Analyses of a Multichiral Drug Molecule: The development of new chemical entities with high chemical and chiral purity is a regulatory expectation in new drug development (18–20). This case study focuses on method improvements of an existing composite HPLC assay (potency and impurities) of a drug molecule with three chiral centres. For the process development of these multichiral molecules, numerous analytical methods capable of separating all stereoisomers (enantiomers and diastereomers) are quickly developed to assess and control the stereochemistry of raw materials, intermediates, and the final API. While chiral HPLC methods are needed for the determination of enantiomers (20), diastereomers are the more likely process impurities for these multichiral molecules, particularly if epimerization occurs during the synthetic process. Achiral reversed-phase methods have the advantage to assess both the stereochemical (diastereomeric content) and overall chemical purity of the API with high-efficiency in a single run. In many cases, these achiral methods become the primary stability-indicating QC methods for these multichiral APIs.
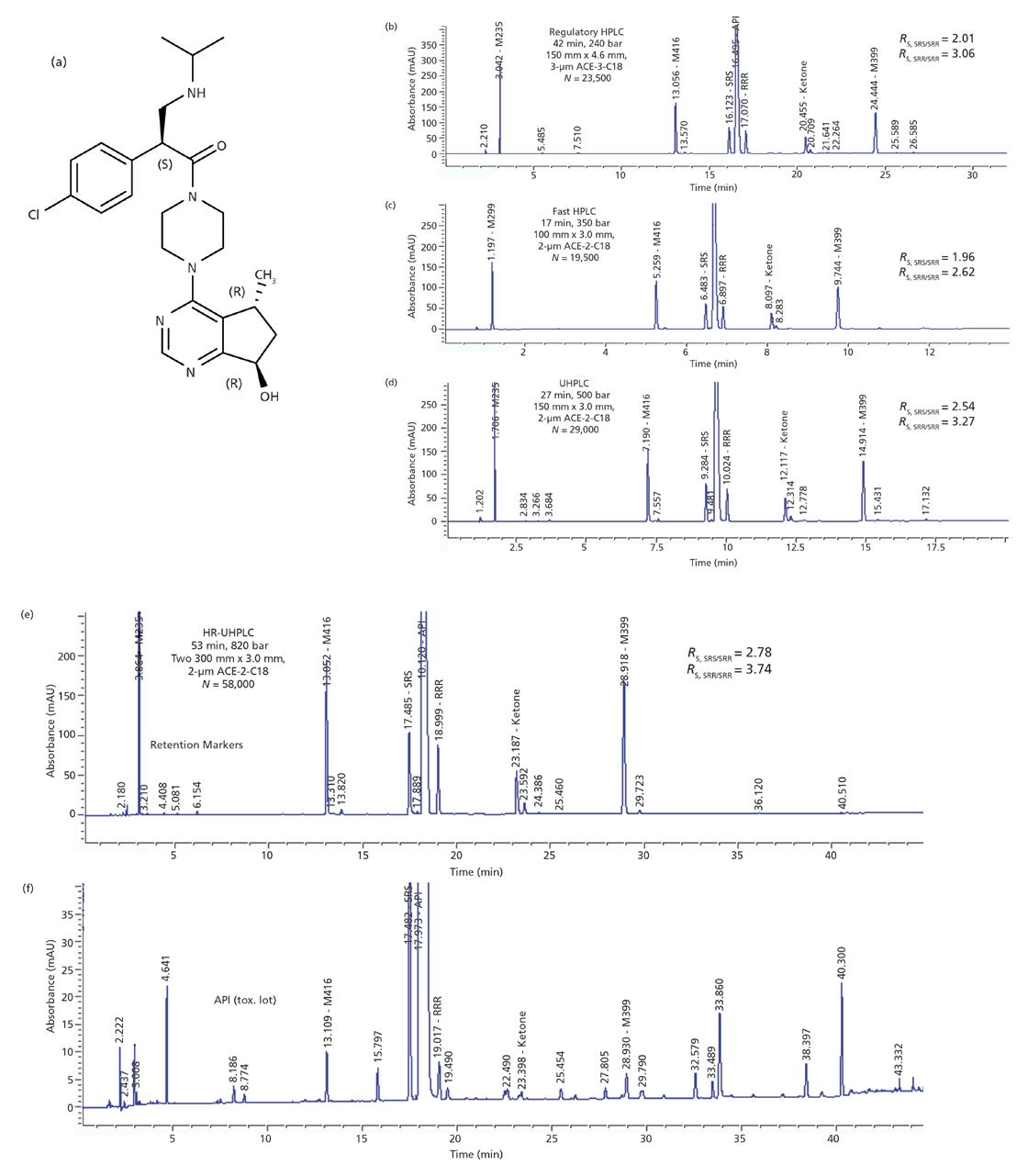
Figure 1: (a) Structure of the active pharmaceutical ingredient (API) with three chiral centres in case study 1. (b)–(f): Comparative chromatograms of a retention marker solution containing an API (0.5 mg/mL) and spiked impurities analyzed using identical mobile phases on an Agilent 1290 binary UHPLC system by various methods: (b) regulatory HPLC, (c) fast HPLC, (d) UHPLC, and (e) high-resolution-UHPLC. Run time, operating pressure, column, plate count, and resolution values of the diastereomers are shown in the figure insets. HPLC conditions: (b) regulatory HPLC method: mobile-phase A: 20 mM ammonium formate at pH 3.7, mobile-phase B: 0.05% formic acid in acetonitrile; flow rate: 1.0 mL/min at 30 °C; gradient programme: 5–15% B in 5 min, 15–40% B in 25 min, 40–90% B in 10 min, 90% B in 3 min, 90–5% B in 0.1 min; detection: 280 nm; injection volume = 10 μL. (c) Fast HPLC method: flow rate: 0.8 mL/min at 40 °C; gradient programme: 5–15% B in 2 min, 15–40% B in 10 min, 40–90% B in 1 min, 90% B in 2 min, 90–5% B in 0.1 min; injection volume = 3 μL. (d) UHPLC method: flow rate: 0.8 mL/min at 40 °C; gradient programme: 5–15% B in 2 min, 15–40% B in 18 min, 40–90% B in 3 min, 90% B in 2 min, 90–5% B in 0.1 min; injection volume = 5 μL. (e) High-resolution-UHPLC method: flow rate: 0.8 mL/min at 40 °C; gradient programme: 5–15% B in 2 min, 15–40% B in 36 min, 40–90% B in 6 min, 90% B in 4 min, 90–5% B in 0.1 min; injection volume = 10 μL. (f) High resolution UHPLC chromatogram of an early API lot used for toxicological assessment using conditions in Figure 1(e). Sample preparation procedure for the API: dissolve 25 mg of API in 50-mL volumetric flask with mobile-phase A.
The drug molecule in this first case study shown in Figure 1(a) has three chiral centres and an absolute configuration of SRR. This molecule has a total of eight potential stereoisomers including the enantiomer (RSS) and three diastereomeric pairs (SRS/RSR, RRR/SSS, and SSR/RRS). Figure 1(b) shows the conventional HPLC method developed several years ago that separates the API (SRR) from the other diastereomers (SRS and RRR) and all expected impurities (M235, M416, ketone [M456], and M399, designated by their mass spectrometric parent ions). Only two diastereomers (SRS and RRR) were found in the actual API synthesized via asymmetric stereospecific processes. The third diastereomer, SSR, which is eluted between SRS and the API, was not found above reportable levels. To maximize the resolution around the API peak where most of the isomers tend to be eluted, a multisegment gradient programme is used. The main component is eluted towards the end of a shallow gradient of 15–40% mobile-phase B in 25 min (21). This shallow gradient segment is preceded by a 5-min gradient segment (5–15% B) to retain a hydrolytic polar degradant (M235) and is followed by a steep 3-min purging gradient segment. This existing HPLC method, shown in Figure 1(b), uses an 150 mm × 4.6 mm, 3.0-µm dp ACE-3-C18 column and has a run time of 42 min. This conventional HPLC method was used successfully for release testing and stability studies during phase 1 of clinical development.
With the advent of UHPLC equipment and the availability of a 2-µm version of the ACE-3-C18 bonded phase material, we saw an opportunity to improve method performance for higher speed or resolution. Results are documented in Figures 1(c), 1(d), and 1(e) with run time, operating pressure, column details, plate count, and resolution values of the diastereomers shown in the insets. Note that all plate counts (N) reported were measured and reported by the column manufacturers under isocratic conditions. These isocratic plate numbers are included here as reference parameters for column efficiency. It should be noted that the actual peak resolutions obtained under gradient conditions are dependent on N as well as selectivity factors (column or mobile phase) and operating parameters (for example, flow rate, gradient time [tG], gradient range, and column temperature) (4,5). Peak capacity, a more useful parameter for comparing performance in gradient analysis (5,15), was not measured or reported here because single linear gradients were not used.
Here is the sequence of method improvement steps performed in our study. First, we developed a faster (run time = 17 min), but equivalent HPLC method using a 100 mm × 3.0 mm, 2-µm dp ACE-2-C18 column, shown in Figure 1(c). A column internal diameter of 3.0 mm was selected versus 2.1 mm because it has ~10% higher efficiency (isocratic column plate count, N = 19,700 versus 17,000 plates) and is less susceptible to extracolumn band broadening effects. Identical mobile phases and gradient segments of the original HPLC method were used with a more optimum flow rate (0.8 mL/min). We were able to use this faster 17-min method successfully for all non-good manufacturing practices (GMP) studies for phase 2 of clinical formulation development.
Second, we increased resolution by using the longer (150 mm) ACE-2-C18 column. Results are shown in Figure 1(d) (using one column) and Figure 1(e) (using two coupled columns) with analysis times of 27 and 53 min, and operating pressures of 500 and 820 bar, respectively. As expected, these UHPLC methods display significantly higher resolution particularly in the API region of the chromatogram because of higher column efficiencies and appropriate operating conditions (tG scale to column length). Resolution values (Rs, 5σ peak width) are increased substantially for SRS/SRR (API) from 2.01 (from the original HPLC method, Figure 1[b]) to 2.54 (Figure 1[d]) and 2.78 (Figure 1[e]), respectively. Similarly, Rs SRR/RRR values are increased from 3.06 to 3.27 and 3.74, respectively. Figure 1(f) shows the chromatogram and the impurity profile of an API lot used in an early toxicological evaluation. This API lot contains many impurity peaks as is typical for early-phase development lots used for toxicological assessments. Note that the traditional geometrical scaling approach (22,23) was not used here as our goal was to demonstrate improved resolution of all expected impurities in the shortest time for routine testing.
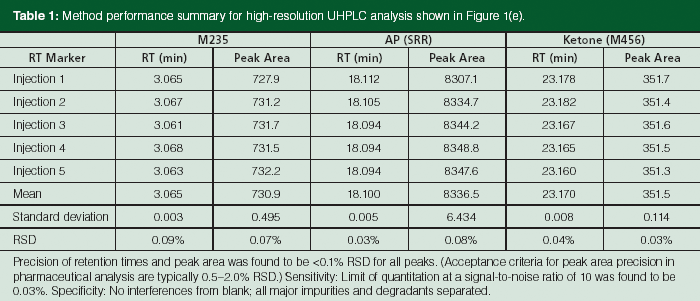
Table 1: Method performance summary for high-resolution UHPLC analysis shown in Figure 1(e).
Method performance data for repeated injections using UHPLC conditions in Figure 1(e) are shown in Table 1, indicating excellent precision (relative standard deviation [RSD] < 0.1% for both retention time and peak area), specificity (separation of all expected impurities including diastereomers), sensitivity (limit of quantitation of 0.03%), and linearity performance (coefficient of linear correlation, R = 1.000 for levels of 50–150% for the API). These excellent performance data, similar to those reported by Deconinck and colleagues (24), demonstrated that modern UHPLC is acceptable for QC applications.
Lessons learned in this case study were as follows:
- Achiral reversed-phase liquid chromatography (LC) methods are useful to assess diastereomeric and chemical purity of molecules with multiple chiral centres in a single run. Furthermore, a multisegment gradient with a shallow middle segment can be used to maximize resolution around the API peak where isomers tend to be eluted.
- The use of long or coupled columns packed with small particles and relatively long tG yields improved resolution of the sample in general and the diastereomers in particular. The use of a 3.0-mm i.d. column versus the 2.1-mm i.d. counterparts appears to be preferable for QC applications because of higher column efficiency and easier transition for users familiar with the operation of 4.6-mm i.d. columns (2–5).
- Excellent method performance data (precision, specificity, sensitivity, and linearity) were demonstrated for this high-resolution UHPLC separation, indicating that UHPLC is compatible with QC analysis of complex pharmaceuticals.
Case Study 2: High-Resolution Stability-Indicating Assay of a Combination Drug Product with Multiple APIs: The second case study shows the results of UHPLC method development efforts for a combination drug product (Stribild oral tablet for the human immunodeficiency virus [HIV] treatment) containing four APIs (elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate). Combination products are becoming increasingly popular for many therapeutic areas and for generic products. Since each API can have its own set of impurities and degradants, the use of HPLC with small-particle columns offers a clear advantage in providing adequate resolution of all these related substances in complex drug products. Figure 2 shows the chromatogram of the final UHPLC method of a mixture of the four APIs spiked with their respective set of impurities (at 0.05–2% levels in a retention marker solution), totalling more than 60 components.
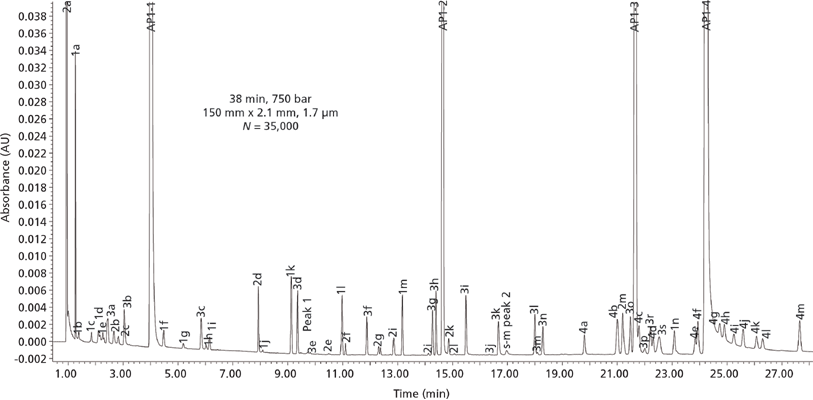
Figure 2: High-resolution UHPLC chromatograms of the retention time marker solution containing the four APIs (labelled 1 to 4) present in the Stribild oral tablet with their "spiked" respective impurities (labelled with letter designation with their respective API). The four APIs are elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate. UHPLC column: 150 mm × 2.1 mm, 1.7-µm Waters Acquity Shield RP18; mobile-phase A: pH 6.2 buffer; mobile-phase B: acetonitrile; flow rate: 0.34 mL/min at 30 °C; gradient programme: an optimized five-segment gradient programme; detection: 240 nm.
The final UHPLC method conditions were derived from an earlier specific, yet nonrobust method. The initial method was developed by the traditional, one factor at a time approach (4–6) over a 6-month period. The elements contributing to the nonrobustness of the initial method were identified as the complex mobile phases (mobile-phase A = buffer at pH 6.0 spiked with formic acid; mobile-phase B = a mixture of acetonitrile, tetrahydrofuran, and mobile-phase A), and a complicated 10-segment gradient profile including a steep segment followed by a long, almost isocratic hold. This initial method suffered from high variability of retention times for several peaks sensitive to pH and gradient conditions. To eliminate this method robustness issue, it was deemed necessary to redevelop the method using simpler mobile phases and with an optimized pH, and a less complex gradient profile.
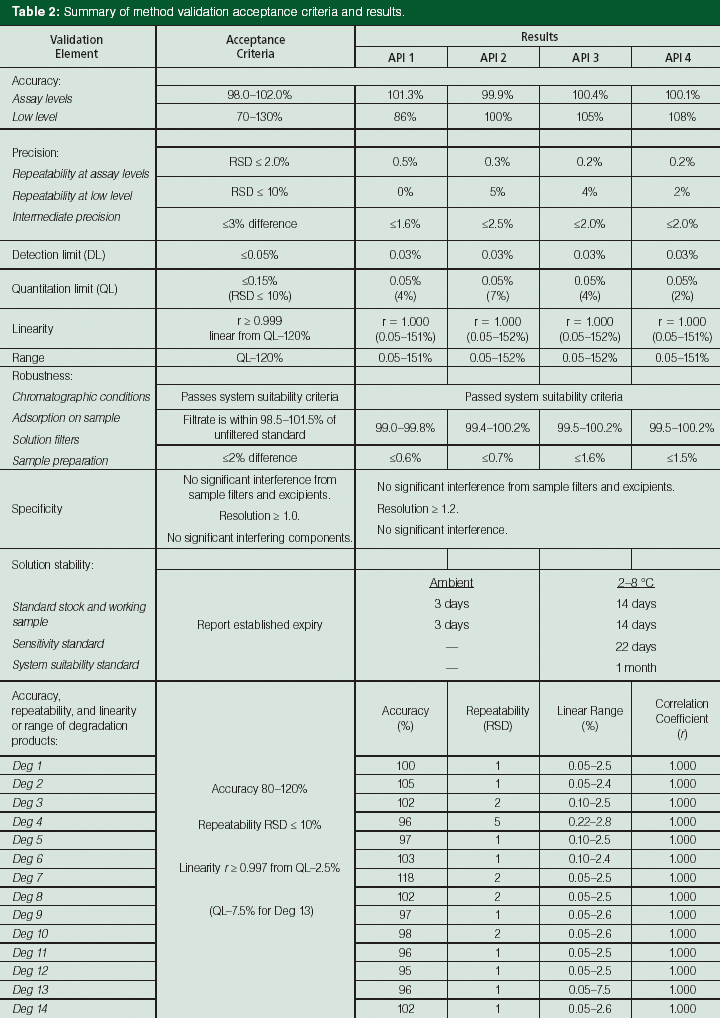
Table 2: Summary of method validation acceptance criteria and results.
This challenging method redevelopment process occurred during phase 3 drug product development and involved the selection of simpler mobile phases (buffer at pH 6.2 as mobile-phase A and acetonitrile as mobile-phase B) and a systematic method optimization process facilitated by a design of experiments (DoE) automation software (2,25). The software allowed a detailed exploration of the multivariate design space and controlling factors in two separate DoE studies (flow rate [0.2–0.45 mL/min], pH of mobile phase A [6.2 ± 0.4 pH units], total gradient time [30 ± 10 min], and column temperature [300 °C ± 100 °C] as well as the slopes of the gradient segments). The results of these systematic experiments provided a better understanding of these factors and the maximization of resolution of several critical pairs in this complex mixture. The resulting UHPLC method was successfully validated according to the International Conference on Harmonisation (ICH) guideline (26), and is used routinely by QC for release testing and stability studies. A summary of method validation acceptance criteria and results are shown in Table 2. The validation data for the 14 expected degradation products, each at a low level, are included in the method validation summary. Process related impurities are controlled in the APIs, and are not typically reported (only monitored) using drug product methods as per ICH guidelines (19). These data indicated that the UHPLC method is suitable for its intended use - the analysis of this complex drug product with multiple APIs.
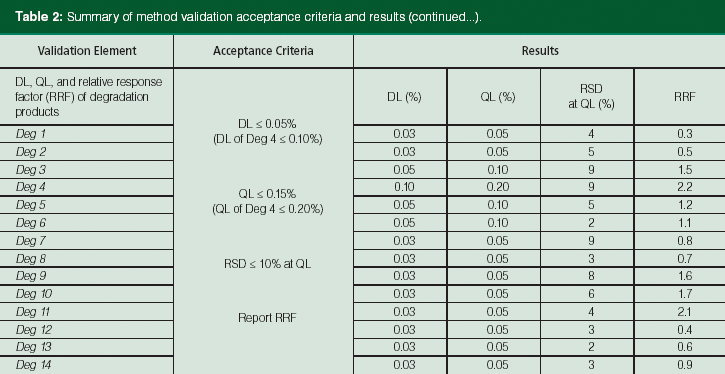
Table 2: Summary of method validation acceptance criteria and results (continued...).
Lessons learned from this method re-development case study were as follows:
- The use of simpler mobile phases, with a more optimum pH and gradient profile in the final UHPLC method was found to improve method performance, robustness, and transferability.
- The use of automated DoE software expedited the method optimization process and allowed the selection of the final conditions in a relatively short time (~2 weeks).
- The final high-resolution UHPLC method for this complex combination drug product was successfully validated and found to be robust for routine QC testing with UHPLC equipment of the same brand and configuration.
Potential Issues of UHPLC for Regulated Pharmaceutical Analysis: Potential issues of UHPLC in pharmaceutical analysis have been reviewed (8,9,27). Many have been mitigated by improved equipment design (that is, peak area imprecision) or judicious selection of system configurations (for example, larger mixer to improve mobile phase blending) (8,27). The effects of viscous heating caused by operating a sub-2-µm column under high pressure are generally well understood (22,28,29). The deleterious effect on column efficiencies by radial thermal gradient effects do not appear to be significant under adiabatic conditions in still air column ovens (<10%). However, axial heating can increase the temperatures at column outlet ends by as much as 20 °C, causing lower retention times and potential selectivity changes. These effects may be problematic for UHPLC method transfer, particularly across different system platforms. In general, method transfer of high-resolution analytical methods can be particularly challenging because of differences between columns (lot-to-lot variation) and system configurations (dwell volumes, design of column oven, and pump or autosampler design) (9), even when equipment from the same vendor is used (22,28).
Another consideration is the lack of control of UHPLC systems by the existing chromatography data system (CDS), as the time lag can be substantial if the UHPLC and the CDS are from different vendors (for example, >12 months in one case for a common CDS). In addition, significant training may be required for operators to become familiar with new UHPLC technologies and operating nuances in dealing with the smaller columns and high pressures. Nevertheless, the ability to accurately analyze very complex samples, an unmet need for conventional HPLC, does provide a welcomed benefit for UHPLC in pharmaceutical analysis.
Concluding Remarks
While HPLC is currently meeting most of the needs in regulatory testing of pharmaceutical samples, the advent of UHPLC equipment and columns provides a unique opportunity for more satisfactory analyses of complex pharmaceutical samples in both research and QC applications. Case studies presented here include the analyses of molecules with multiple chiral centres and complex drug products containing multiple active ingredients. We demonstrated that high-resolution chromatography with excellent method performance can be achieved in these cases using 150-mm columns packed with sub-2-µm particles and run times of 30–60 min. Potential technical issues including viscous heating effects, pump blending, method transfer, and other QC related requirements have been discussed. Most issues can be mitigated through planning and judicious selection of system configurations or operating conditions.
The analytical scientist in pharmaceutical laboratories expends significant efforts in the development of stability-indicating methods to support research and for QC applications. The continuing advances in UHPLC technologies clearly offer a more satisfactory solution in the analysis of complex pharmaceuticals.
Acknowledgements
The authors thank Mohammad Al-Sayah, Dawen Kou, Cadapakam Venkatramani, Eileen Zhao, and Beiwei Lin of Genentech; John Dolan from LC Resources; Ross Woods of the University of Texas at Arlington; Ron Majors, Monika Dittmann, and Klaus Witt from Agilent Technologies; Jeff Layne from Phenomenex; Pilar Franco of Chiral Technologies; Raphael Ornaf of Vertex pharmaceuticals; and Tom Waeghe from MacMod Analytical for helpful discussions and suggestions.
Michael W. Dong and Nik P. Chetwyn are with Small Molecule Analytical Chemistry and Quality Control at Genentech in South San Francisco, California, USA.
Davy Guillarme and Szabolcs Fekete are with the School of Pharmaceutical Sciences at the University of Geneva, University of Lausanne in Geneva, Switzerland.
Rozaliya Rangelova, Jody Richards, and Dan Prudhomme are with Analytical Sciences, Gilead Sciences in Foster City, California, USA.
References
(1) J.E. MacNair, K.C. Lewis, and J.W. Jorgenson, Anal. Chem. 69, 983–989 (1997).
(2) D. Guillarme and M.W. Dong, Amer. Pharm. Rev.16(4), 36–43 (2013).
(3) N. Wu, J.A. Lippert, and M.L. Lee, J. Chromatogr. A911, 1–12 (2001).
(4) L.R. Snyder, J.J. Kirkland, and J.W. Dolan, Introduction to Modern Liquid Chromatography (John Wiley & Sons, New York, USA, 2009).
(5) M.W. Dong, Modern HPLC for Practicing Scientists (Wiley-Interscience, Hoboken, New Jersey, USA, 2006).
(6) HPLC for Pharmaceutical Scientists, Y.V. Kazakevich and R. LoBrutto, Eds. (Wiley, Hoboken, New Jersey, USA, 2007).
(7) UHPLC in Life Sciences, D. Guillarme, J.-L. Veuthey, and R.M. Smith, Eds. (Royal Society of Chemistry, Cambridge, UK, 2012).
(8) M.W. Dong, LCGC North Am.25(7), 656–666 (2007).
(9) K.J. Fountain and P.C. Iraneta in UHPLC in Life Sciences, D. Guillarme, J.-L. Veuthey, and R.M. Smith, Eds. (Royal Society of Chemistry, Cambridge, UK, 2012), pp. 20–64.
(10) N. Wu and A.M. Clausen, J. Sep. Sci.30, 1167–1182 (2007).
(11) U.D. Neue, M. Kele, B. Bunner, A. Kromidas, T. Dourdeville, J.R. Mazzeo, E.S. Grumbach, S. Serpa, T.E. Wheat, P. Hong, and M. Gilar in Advances in Chromatogr. Volume 48, E. Grushka and N. Grinberg, Eds. (CRC Press, Boca Raton, Florida, USA, 2009), pp. 99–143.
(12) D. Cabooter and G. Desmet in UHPLC in Life Sciences, D. Guillarme, J.-L. Veuthey, and R.M. Smith, Eds. (Royal Society of Chemistry, Cambridge, UK, 2012), pp. 1–25.
(13) P. Sandra and G. Vanhoenacker, J. Sep. Sci.30, 241–244 (2007).
(14) D. Guillarme, J. Ruta, S. Rudaz, and J.-L. Veuthey, Anal. Bioanal. Chem.396, 1069–1082 (2010).
(15) D. Guillarme, E. Grata, G. Glauser, J.-L. Wolfender, J-L. Veuthey, and S. Rudaz, J. Chromatogr. A1216, 3232–3243 (2009).
(16) D.T. Nguyen, D. Guillarme, S. Heinisch, M.-P. Barrioulet, J.-L. Rocca, S. Rudaz, and J.-L. Veuthey, J. Chromatogr. A1167, 76–84 (2007).
(17) R.S. Plumb, P. Rainville, B.W. Smith, K.A. Johnson, J. Castro-Perez, I.D. Wilson, and J.K. Nicholson, Anal. Chem.78, 7278–7283 (2006).
(18) International Conference on Harmonisation, ICH Q3A (R2), Harmonized Tripartite Guideline, Impurities in New Drug Substances (ICH Expert Working Group, Geneva, Switzerland, 25 October 2006).
(19) International Conference on Harmonisation, ICH Q3B (R2), Harmonized Tripartite Guideline, Impurities in New Drug Products (ICH Expert Working Group, Geneva, Switzerland, 2 June 2006).
(20) V.D. Heyden in Handbook of Pharmaceutical Analysis by HPLC, S. Ahuja and M.W. Dong, Eds. (Elsevier/Academic Press, Amsterdam, Netherlands, 2005), pp. 447–489.
(21) M.W. Dong, LCGC North Am.31(8), 612–621 (2013).
(22) M.W. Dong, LCGC North Am.31(10), 868–880 (2013).
(23) B. Debrus, E. Rozet, P. Hubert, J.-L.Veuthey, S. Rudaz, and D. Guillarme in UHPLC in Life Sciences, D. Guillarme, J.-L. Veuthey, and R.M. Smith, Eds. (Royal Society of Chemistry, Cambridge, UK, 2012), pp. 67–98.
(24) E. Deconinck, P.Y. Sacré, S. Baudewyns, P. Courselle, and J. De Beer, J. Pharm. Biomed. Anal. 56, 200–209 (2011).
(25) Y. Li, G.T. Terfloth, and A.S. Kord, Amer. Pharm. Rev.12, 87–95 (2009).
(26) International Conference on Harmonisation, ICH Q2 (R1), Harmonised Tripartite Guideline, Validation of Analytical Procedures, (ICH Expert Working Group, Geneva, Switzerland, November 2005).
(27) M.W. Dong in Chromatography: A Science of Discovery, R.L. Wixom and C.W. Gehrke, Eds. (Wiley, Hoboken, New Jersey, USA, 2010), pp. 328–332.
(28) L. Nováková, J.-L. Veuthey, and D. Guillarme, J. Chromatogr. A1218, 7971–7981 (2011).
(29) F. Gritti and G. Guiochon, Anal. Chem.80, 5009–5020 (2008).


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










