Positive Impacts of HPLC Innovations on Clinical Diagnostic Analysis
Special Issues
As clinical diagnostic assays move to LC–MS–MS, the emphasis has turned to emerging stationary phases that use alternative mechanisms of retention to separate the analyte–interference critical pairs.
Michael J.P. Wright and Sophie Hepburn, Department of Clinical Chemistry and Endocrinology, Prince of Wales Hospital, Sydney, Australia.
The last decade has seen a series of advances in the field of liquid chromatography that have resulted in improvements for many clinical diagnostic services. These innovations have included the expansion of superficially porous particle columns, new or improved stationary phase options, and user-friendly multipleâchannel high performance liquid chromatography (HPLC) instrument options that allow sequential analysis - a boon for low- and moderate-throughput laboratories with limited hardware. As a result, diagnostic services are able to offer faster turnaround times and measure analytes in patient types and disease states that were previously problematic. This article presents examples of the impact these innovations have had in a number of hospital settings.
Chromatographic techniques (including liquid, gas, and thin-layer chromatography), have been used for decades in specialist clinical laboratories for the separation and (semi-) quantitation of established biomarkers. High performance liquid chromatography (HPLC) emerged as the most useful technique in the clinical field and has been commonplace for biospecimen analysis since the 1970s (1). Over the last 20 years there has been a shift towards mass spectrometry (MS) detection rather than conventional detection methods (ultraviolet [UV], fluorescence, and electrochemical) (2). This shift was driven by the uptake of liquid chromatography coupled to tandem MS (LC–MS–MS) in newborn screening and therapeutic drug monitoring laboratories where the advantages of reduced turnaround times and a simplified workflow were paramount. In recent years, LC–MS–MS has also started to replace gas chromatography (GC)–MS and immunoassay methods for vitamins, hormones, and metabolites. This trend is mainly due to the superior selectivity and adequate sensitivity of LC–MS–MS compared to immunoassays (3) and the higher throughput and capacity compared to GC–MS. The result is that, today, many specialist clinical laboratories continue to use a dwindling number of wellâestablished GC, GC–MS,
HPLC–UV, HPLC–electrochemical detection (ECD), and HPLC–fluorescence detection (FD) methods and an everâincreasing number of
LC–MS–MS methods.
Diagnostic clinical chemistry departments, particularly those within the public health system, usually receive budgetary funding on a reimbursement per result basis. In these laboratories, the majority of sample analysis is performed on large, fully automated colorimetric, nephelometric–turbidimetric, or ligand binding assay instruments, which are often acquired from vendors via reagent rental or cost-per-assay agreements. This means that the vendor provides the instrument in exchange for a guaranteed purchase of reagents per year, or alternatively, the clinical laboratory must pay the vendor a specified amount per test processed. These arrangements work well because they are suitable for the budget model and remove the considerable capital outlay of purchasing equipment. In addition, because the average contract expires after five years, laboratories can keep up with technological advances via the tendering process. This remuneration scenario is not the norm for chromatography and MS equipment vendors supplying instrumentation to specialist clinical chemistry laboratories. As a result, obtaining the capital funds to purchase LC–MS–MS instrumentation often requires detailed business cases demonstrating costâeffectiveness to accompany the predicted clinical benefit of using this technology.
Despite a history spanning more than 40 years, chromatographic challenges are commonplace in clinical diagnostic analysis, even with the improved selectivity offered by MS detection. Interferences that prevent result reporting can arise from specific patient groups because of age, sex, diet, disease states, and drug regimens. The complexity of samples from various patient populations can necessitate more than one chromatographic method for the analysis of a target biomarker to enable measurement across all patient subgroups and allow for confirmatory testing when unusual interferents are present. This need places a great deal of emphasis on stationary-phase options that explore different retention mechanisms, and emerging phases on the market are met with great enthusiasm.
Rapid and Robust Analysis without the Back Pressure
Many HPLC services provided in specialist centres employ established assays using older instrumentation with back-pressure limits of 5000 psi or less. In the past, the relatively long chromatographic run times were not of concern because sample numbers were low. However, the ever-increasing workload (typically ≥10% expansion year on year) has meant that chromatographic run time has become a limiting factor. Unfortunately, the costs of replacing instrumentation with ultrahighâpressure liquid chromatography (UHPLC) equipment capable of recognizing the gains of sub-2-µm dp columns can be prohibitive.
An important development in column technology was the emergence of >2-µm superficially porous silica particles (SPPs) that can provide increased efficiency without the same back-pressure gains as those seen with sub-2âµm fully porous particle columns. For established clinical HPLC assays, for example, serum vitamins A and E (HPLC–UV) and urine catecholamines, metanephrines, and 5-hydroxyindoleacetic acid (HPLC–ECD), the introduction of higher efficiency SPPs enabled the use of shorter columns to produce very similar chromatographic separation with greatly reduced run times and minimal changes in back pressure (Figure 1). In many cases, this increased the capacity of existing HPLC instrumentation 3–4-fold. Interestingly, the main limitation in accelerating the chromatography further is no longer the back pressure, but rather a combination of other factors including autosampler operating speed, detector cell volume, and the data collection rate limit of the detector.
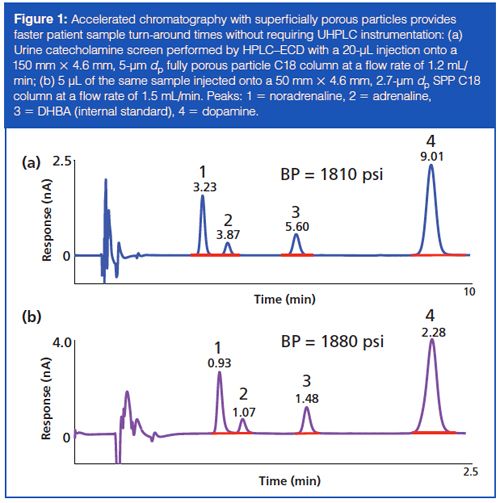
Accelerated chromatography can certainly aid in improving patient sample turn-around times; however, ideally it should not come at the expense of robustness of the method. System blockages cause delays in patient result reporting while the instrument undergoes troubleshooting and repairs. One trade-off seen with UHPLC systems fitted with sub-2-µm dp columns, when compared to a HPLC system fitted with a larger dp column, is the requirement for cleaner, particulate-free mobile phases and more exhaustive sample preparations before injection to prevent blockages. In a high-throughput clinical laboratory these extra sample preparation requirements can be an additional burden on staffing and workflow. SPP columns with >2-µm particles provide an intermediate solution.
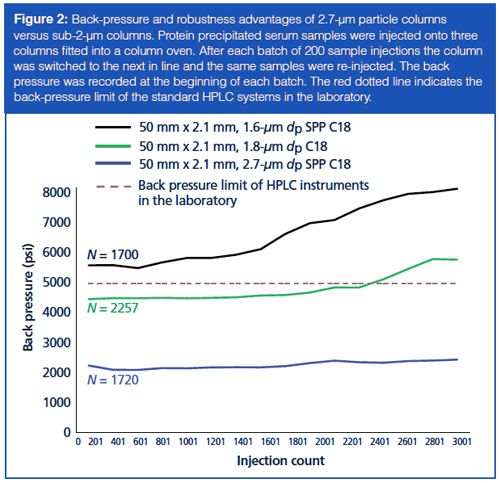
To assess robustness we performed the following experiment: 100 µL of serum samples were protein precipitated by the addition of 25 µL of 0.2 mol/L zinc sulphate followed by 200 µL of methanol. The resulting solution was passed through a 0.45âµm 96-well Multiscreen Solvinert filter plate (Millipore) before being loaded onto a Nexera series UHPLC system (Shimadzu) coupled to an API 6500QTRAP mass spectrometer (Sciex). The three columns under evaluation were fitted into the column oven using multiport selection valves. Next, 20 µL of each prepared sample was injected onto the column and after each batch of 200 sample injections the column was switched to the next in line and the process was repeated. Mobile-phase A was 0.1% formic acid in water and B was 0.1% formic acid in methanol. A rapid gradient of 50–100% B was performed over 1 min at 0.4 mL/min, followed by 100% B at 1 mL/min for 1 min, and then 50% B at 1 mL/min for 0.5 min. The back pressure was recorded at the beginning of each batch and column efficiency was determined by an injection of a system suitability test solution containing testosterone. As expected, the subâ2âµm dp columns initially generated a higher back pressure than the 2.7 µm dp column; however, the efficiency (N) was dependent on column type rather than purely the particle size (Figure 2). Back pressure increased for all columns as the injection number increased, but this elevation was more marked in those with sub-2-µm dp particles. Unlike the UHPLC system used in this study, a number of the HPLC systems in the laboratory have a back-pressure limit of 5000 psi, so for this example only the 2.7-µm dp column would be suitable for all instruments over a large number of injections.
Innovations in Stationary Phases
The move towards LC–MS–MS analysis in clinical chemistry has provided many benefits; however, challenges involving chromatographic separation of similar compounds remain. With the number of solvents and additives now limited to those that are considered “MS friendly” (that is, volatile, proton donating, or accepting, do not form unwanted adducts), the selection of stationary phase has taken on increased importance (4). Chromatography is required not only for the removal of interferents causing ion suppression, particularly salts and phospholipids present in blood and urine, but also for the separation of isobaric compounds that share the mass transitions used for quantitation. Alkyl-bonded stationary phases have been the traditional mainstay of clinical HPLC separations with mobile-phase buffers and ionâpair reagents providing the additional selectivity required. As assays move to LC–MS–MS, the emphasis has turned to emerging stationary phases that use alternative mechanisms of retention to separate the analyte–interference critical pairs.
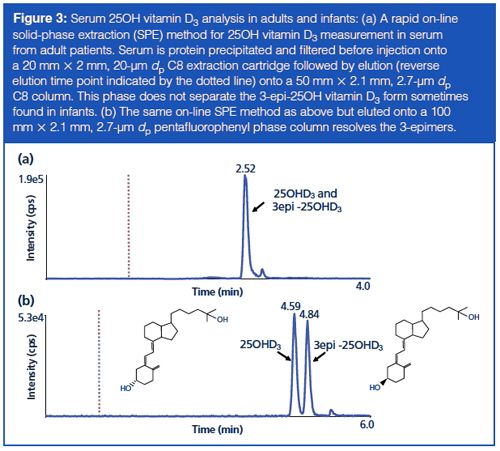
Serum 25OH vitamin D3 measurement has seen substantial growth in clinical chemistry laboratories over the past 10 years; with test requests having increased approximately twofold per year, every year. One of the challenges presented by the measurement of this biomarker with LC–MS–MS is separating the C3-epimeric forms often found in samples from infants. The 3-epi-25OH form of vitamin D3 is thought to be an inactive or possibly a suppressing form of 25OH vitamin D3 with the general consensus that it should be separated for measurement of 25OH vitamin D3 in infant patient samples (5). Because the C3-epimeric forms share the same precursor–product ion mass spectra they need to be separated before arrival at the mass spectrometer ion source. Many LC–MS–MS methods in the literature were designed for older patients and used C8 or C18 stationary phases that were unable to resolve this critical pair. In the past, chromatography using cyano stationary phases were utilized but limited selectivity of the phase resulted in run times of 18–45 min, which were too long to be feasible for the volume of test requests (6). The development of pentafluorophenyl (PFP) phases has shown improved selectivity for 25OH vitamin D3 and 3-epi-25OH vitamin D3, enabling quantitation of the target analyte in this patient group (Figure 3), either as a secondary method for infant samples or, if time permits, as a frontâline analysis for all samples (7).
Issues with isobaric interferences in clinical LC–MS–MS can be exacerbated when the compounds are extremely polar and difficult to separate by most hydrophilicâinteraction chromatography (HILIC) phases. The measurement of Kreb cycle compounds demonstrates this scenario where the critical pairs of isocitrate–citrate and succinate–methylmalonic acid need to be resolved. The evolution and diversification of phases that incorporate an embedded polar group (EPG) with an alkyl ligand has produced stationary phases with a wide range of chromatographic properties that are capable of operating at 100% aqueous mobile phases (8). In this example, an EPG stationary phase, operated under aqueous conditions, enabled resolution of these critical pairs (Figure 4[a]).
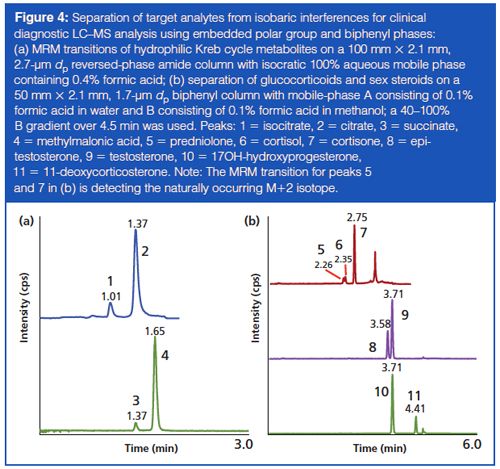
For the measurement of serum steroids such as cortisol, testosterone, or 17-hydroxyprogesterone, the target analyte is part of a large group of closely related endogenous compounds that share a fused ring system of three cyclohexanes and one cyclopentane and where isobaric interferences are common. To further complicate matters, because of their structural similarity, steroids often have similar fragmentation patterns and can also be present at supraphysiological concentrations, for example, when used for treatment. Thus, even for compounds that have slightly different precursor mass-to-charge ratios (m/z), the possibility of interference from naturally occurring isotopes of steroids with smaller m/z values have to be taken into consideration. This is particularly true when developing LC–MS–MS methods for the analysis of samples from certain patient groups, such as those with steroidogenesis defects. The emergence of biphenyl phases introduces separation mechanisms such as shape selectivity and π-π interactions while providing a greater amount of hydrophobic retention than seen with traditional phenyl phases. The use of a biphenyl phase enables the separation of common isobaric steroid interferences, such as those outlined in Figure 4(b).
Leveraging the Most Out of Instrumentation
For small to moderate-sized specialist clinical laboratories (500–5000 patient samples/week) the LC–MS–MS workflow consists of numerous applications where small batches of patient samples are run on a regular basis (daily or weekly). Often these applications rely on different mobile and stationary phases to achieve the chromatographic selectivity required. To leverage the capacity of the LC–MS–MS instruments to achieve favourable cost-effectiveness, the systems should run continuously with an automated process for changing from one method to another without intervention by staff.
HPLC systems that incorporate selection-valve configurations allowing multiple mobile phase and column combinations to be run simultaneously have existed for some time in research and assay development laboratories. However, because of reliability issues, complicated software, concerns regarding service-support, and a lack of experienced operators in the laboratories themselves, these instruments were not regularly promoted to clinical diagnostic laboratories. This situation has changed in recent years with a number of LC–MS–MS vendors providing simpler instrument setâups with numerous on-board mobile phases and columns easily controlled via instrument software.
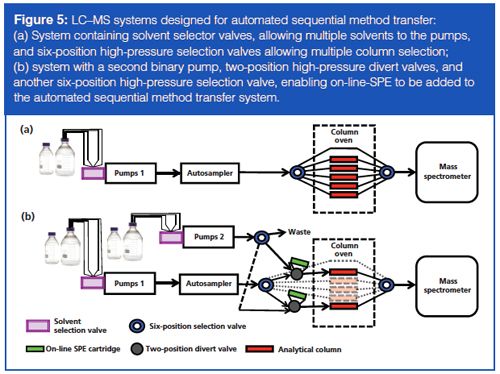
Figure 5(a) illustrates a system where binary pumps have solventâselection valves attached allowing multiple mobile phases (typically, four or six) to each pump. In our laboratory, one of the lines running to each pump is reserved for a “cleaning solvent” of 50% methanol. The column oven houses two seven-port, six-position selection valves allowing availability of up to six columns without system reconfiguration. In this setup, one column option is sacrificed for a direct line for use during conditioning steps. For sequential analysis of multiple assays, all sample preparation is completed during normal working hours and the prepared samples are loaded into the autosampler by the end of the day. The batches of samples are submitted together with conditioning batches introduced between different assays to run overnight as follows:
- The first batch is run using Method 1: column 1 and mobile-phase A1 and B1.
- A “conditioning batch” is run using blank samples injected utilizing General Conditioning Method*: direct line (no column) and mobileâphase A4 and B4 (cleaning solvent) - this step purges the system with 50% methanol, removing the mobile phases from Method 1 and thus preventing the possibility of incompatible mobile phases mixing from two different methods.
- A second conditioning batch is run with blank samples injected utilizing Method 2 Conditioning Method*: direct line (no column) and mobileâphase A2 and B2 (Method 2 mobile phases) - This primes the system with the correct mobile phases for Method 2 in preparation for the next column to be switched on-line.
- The second batch is run using Method 2: column 2 and mobileâphase A2 and B2.
- The “conditioning batch” is run again with blank samples injected using General Conditioning Method*: direct line (no column) and mobileâphase A4 and B4 (cleaning solvent).
- A third conditioning batch is run with blank samples injected using Method 3 Conditioning Method*: direct line (no column) and mobileâphase A3 and B3 . . . and so on, with up to five different clinical assay methods running without user intervention.
- *The liquid flow during the various conditioning batches is diverted to waste immediately before the mass spectrometer to prevent fouling of the ion source.
On-line solid-phase extraction (SPE) is a popular technique in clinical diagnostic laboratories because of the labour and cost savings it represents compared to off-line SPE. Adding multiple on-line SPE stations to a system (Figure 5[b]) can be achieved by the introduction of a second set of pumps, fitted with solvent selection valves, and a high-pressure selection valve to direct the flow from these pumps to the switching valve fitted with the on-line SPE cartridge. Again, one mobile-phase channel for each pump is reserved for a “cleaning solvent” of 50% methanol and one option from the selection valve is sent to waste for use during conditioning steps. As with the example described previously, general conditioning and method-specific conditioning batches are submitted between batches to prepare the system with the correct mobile phases, column oven temperatures, and mass spectrometer ion source conditions for the subsequent sample batch.
A clear advantage in running assays sequentially on a single system (or even parallelism in larger laboratories) is that of operation time (uptime) allowing laboratories to provide a 24-h service to users. Priority is obviously given to urgent tests, which can be run during the daytime with rapid reporting of results. However, routine batch tests can run throughout the night to minimize sample congestion during working hours where instruments would be better used for assay development and improvement processes. It also allows LC–MS–MS methods to compete with routine immunoassay analyzers that are common to core testing facilities that often perform 50+ reactions concurrently.
Conclusion
HPLC continues to have widespread applications in clinical laboratories, and, when coupled to MS, it is the preferred method for the measurement of many low-concentration endogenous biomarkers. The advent of SPP introduced higher efficiencies on existing HPLC equipment, enabling the faster run-times required to match the growing sample numbers without the need to purchase expensive UHPLC equipment. In addition, the introduction and expansion of a greater variety of stationary phases to the market has helped to solve many of the chromatographic challenges facing clinical laboratories moving to LC–MS–MS technology. Throughput limitations for LC–MS–MS platforms have been resolved by multiple-channel systems that enable programming of sequential analyses over a 24-h period. Adding on-line SPE to these platforms further streamlines the workflow and introduces cost-savings in a busy clinical setting. These innovations allow several assays to run back to back with a variety of stationary and mobile phases without the need for expensive, and often unfavourable, night-shift schedules for highly skilled staff while also delivering value for money from expensive LC–MS–MS platforms.
References
- C.A. Burtis, J. Chromatogr.52, 97–106 (1970).
- S.K.G. Grebe and R.J. Singh, Clin. Biochem. Rev.32, 5–31 (2011).
- V.M. Carvalho, J. Chromatogr. B 883–884, 50–58 (2012).
- S.R. Needham, P.R. Brown, K. Duff, and D. Bell, J. Chromatogr. A869, 159–170 (2000).
- A. De La Hunty, A.M. Wallace, S. Gibson, H. Viljakainen, C. LambergâAllardt, and M. Ashwell, Br. J. Nutr.104(4), 612–619 (2010).
- K.W. Phinney, M. Bedner, S.S. Tai, V.V. Vamathevan, L.C. Sander, K.E. Sharpless, S.A. Wise, J.H. Yen, R.L. Schleicher, M. Chaudhary-Webb, C.M. Pfeiffer, J.M. Betz, P.M. Coates, and M.F. Picciano, Anal. Chem.84(2), 956–962 (2012).
- C.R. Aurand, D.S. Bell, and M. Wright, Bioanalysis 4(22), 2681–2691 (2012).
- M.R. Euerby and P. Petersson, J. Chromatogr. A1088, 1–15 (2005).
Michael J.P. Wright and Sophie Hepburn are with the SEALS Department of Clinical Chemistry and Endocrinology at the Prince of Wales Hospital in Sydney, Australia. Direct correspondence to: mike.wright333@gmail.com or shepburn_au@yahoo.com

HPLC 2025 Preview: Fundamentally Speaking (Part 1)
May 13th 2025Michael Lämmerhofer from the Institute of Pharmaceutical Sciences, University of Tübingen, Germany, spoke to JFK Huber Lecture Award winner of 2024 Torgny Fornstedt, professor in analytical chemistry and leader of the Fundamental Separation Science Group, Karlstad University, Sweden, about his pioneering work in high performance liquid chromatography (HPLC) with a focus on fundamentals and industrial applications.
Reversed-Phases for LC Deliberately Doped with Positive Charge: Tips and Tricks for Effective Use
May 13th 2025In this month's edition of LC Troubleshooting, Dwight Stoll and his fellow researchers discuss both the benefits (improved peak shape/loading) and challenges (excessive interaction) associated with charge-doped reversed-phase (RP) columns for both analytical and preparative separations.
Determining Ways to Protect Honeybee Colonies with GC–MS
May 13th 2025A study conducted by the Agriculture Research Centre of Giza, Egypt, and Jilin Agricultural University in China, evaluated the efficacy of stinging nettle extract, nettle smoke, and formic acid in the controlling of Varroa mites, a major threat to honeybee colonies, with a focus on mite infestation reduction, honeybee mortality, and biochemical responses. Gas chromatography–mass spectrometry (GC–MS) was used to identify key bioactive compounds in the stinging nettle extract.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











