Latest Advances in Environmental Chiral Applications
Special Issues
Recent advances in chiral stationary phases have enabled higher efficiency and faster separations in studies of the differing enantiomeric activity of pesticides, their environmental transformation, and the degradation of pollutants in general.
Denise Wallworth, Sigma-Aldrich UK, a subsidiary of Merck, Poole, Dorset, UK.
This article provides a brief overview of some of the chiral environmental studies carried out recently that cover the differing enantiomeric activity of pesticides, their environmental transformation, and the degradation of pollutants in general. It highlights some of the recent advances in chiral stationary phases that have enabled higher efficiency and faster separations than previously seen in this area.
While in earlier years environmental applications concentrated mainly on agrochemicals in use, it is now a much broader field covering not just preferential enantioselective activity, but also the influence of microbial population on selective degradation. The impact of excess and metabolized pharmaceuticals is also widely studied along with persistent organic pollutants (POPs), that includes a range of both pesticides and fluorinated organics. There is increasing alarm about these POPs potentially not being fully extracted in waste-treatment plants, affecting both human and fish populations. Monitoring for banned pesticides is also a key activity.
Recent developments in chiral stationary-phase (CSP) technology have principally concerned major advances in smaller particle technology to support these needs. The result is vastly improved chiral column efficiency and selectivity enabling many new applications along with the potential for more comprehensive multicomponent screening. A significant number of applications have used 3-µm particle size versions of both the immobilized and coated polysaccharide CSPs to enable much faster separations. There has been much interest in the utilization of sub-2-µm ultrahigh-pressure liquid chromatography (UHPLC) particles for chiral separations, mostly on brush type and cellulosic CSPs. A recent paper by Gasparrini (1) demonstrated the benefits of both UHPLC and supercritical fluid chromatography (SFC) and the high efficiencies obtained by bonding onto Whelk-O-1 both 1.7-µm porous silica and superficially porous particle (SPP, core–shell) silica. The next phase of new CSP development for this year, however, is very likely to use the recent introduction of a 1.9-µm monodisperse totally porous particle (TPP) (2) that appears to provide new opportunities to increase CSP efficiencies even further. These particles, when bonded with C18, exhibited an extremely low reduced plate height, h, of 1.7 in narrow-bore (2.1 mm i.d.) columns, extremely low when compared to classical porous particles. Gasparrini (3) demonstrated extremely high efficiencies obtained by bonding teicoplanin to TPPs and performed extensive fundamental studies of the CSP, reporting efficiencies of 200,000–250,000 plates/m at the optimum flow rate. Additionally, Armstrong and coworkers (4,5) reported ultrafast separations by bonding cyclofructan, cyclodextrin, and all the macrocyclic chiral selectors to TPPs. Separations in seconds were demonstrated that could, for instance, provide on-line chiral monitoring of asymmetric synthesis. The very significant increase in efficiency should enable the separation of far more complex mixes in addition to being used for two dimensional (2D)-UHPLC for the separation of multichiral centre pesticides in the near future (4).
Environmental Applications
The fast growth in agrochemicals has fueled much research into their environmental impact. It is estimated that 28% of agrochemicals are currently chiral (6), a growth in part because of advances in asymmetric synthesis and process scale simulated moving bed chromatography that has significantly reduced the commercial cost for multitonnage agricultural requirements. Despite this, some 24% of these are applied as a racemate (7), resulting in the potential release of inactive products into the environment. Of the $223 billion global pesticide industry, more than 40% of the products are used in China and the largest proportion of papers published in the last two years reflects this, especially where related to their impact on the important tea production industry.
The fate of pesticides in the environment is expected to be subject to enantioselective biodegradation by microorganisms, possibly in quite a different way compared to microorganisms present in water because of residue matrix binding effects. The differing enantiomeric activities of chiral pesticides, the effect of various microorganisms giving differing modes of degradation, and the resulting unbalancing of the microbiological makeup of the environment are all of great interest. Pharmacologically active compounds - drugs, their metabolites, and illicit drugs - are routinely tested in wastewater, but could also be present in solid waste and sludge providing an additional source of bioavailable uptake. Overall, there are many reasons for increased environmental testing.
Chiral Method Development for Environmental Applications
While chiral separations in environmental applications have generally not kept pace with those for pharmaceutical products, the number of publications has grown considerably in recent years due, in part, to these increasing environmental concerns. CSPs used for environmental separations need to be capable of separating relatively polar molecules. Fortunately, high performance liquid chromatography (HPLC), gas chromatography (GC), and SFC have all proven to be useful. SFC was used for the enantiomeric analysis of the triazole fungicide flutriafol in vegetables, fruits, and soils in 3.5 min using a 3-µm bonded amylose tris(3,5-dimethylphenylcarbamate) CSP in carbon dioxide–methanol. Formic acid in methanol was added post column to enhance mass spectrometry (MS) ionization. Using QuEChERS (quick, easy, cheap, effective, rugged, and safe) for sample preparation, this method provided a limit of quantitation (LOQ) down to 0.41 µg/kg, making it useful for both environmental and food analysis (8). This separation has also been performed by LC–MS using a cellulosic tris(3-chloro-4-methyl phenyl carbamate) phase in 40:60 (v/v) acetonitrile–water giving a limit of detection (LOD) of 15 µg/kg (9). This was found to be a much faster method than using a cellulosic phase under normal-phase conditions. Elution order and configuration were assigned using electronic circular dichroism (ECD) and found to be (R)-(-) for the first eluting enantiomer and (S)-(+) for the second. Linearity and precision was checked in seven different matrices, in preparation for future environmental and food studies. Chiral GC was used for a haloxyfop study (10,11), separating these herbicides as their methyl esters using a custom-made OV170 GC column coated with 15% w/w permethylated beta cyclodextrin (0.1-µm film thickness). The levels of organochlorine pesticides in air and surface water in the Indian Ocean were measured using chiral GC–MS (12) employing EI detection in multiple reaction monitoring (MRM) mode and a 20% tert-butyldimethylsilyl-beta-cyclodextrin CSP dissolved in 15% phenyl-, 85% methylpolysiloxane. Significant decreases in α-HCH and γ-HCH but increases in p,p′-DDT, o,p′âDDT and cis- and trans-chlordane were observed. An example separation of chlordane and HCH is shown in Figure 1.
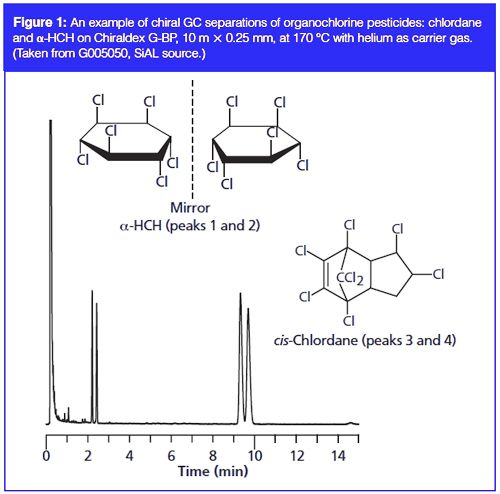
For the monitoring of active pharmaceuticals in wastewater, a method for the simultaneous enantioselective determination of ibuprofen, naproxen, and ketoprofen was developed using LC–MS–MS. The method used a single-step sample treatment based on microextraction with a supramolecular solvent that provided low method detection limits of 0.5–1.2 ng/L. This was optimized and the analytical method validated on a vancomycin bonded 5-µm CSP (13). The method was reported as suitable for using the enantiomeric fraction of ibuprofen as an indicator of the discharge of untreated or poorly treated wastewaters. In contrast, a (nonchromatographic) 14C isotope tracing MS–MS method was used to investigate the fate of the four isomers of IPP, a novel, broad spectrum neonicotinoid insecticide (7). Stereoselective soil binding and the microbial influence on epimer-selective degradation were reported.
Enantioselective Activity
As is well known for chiral pharmaceutical products in biological systems, if a pesticide is a chiral molecule, it is common that one enantiomer carries greater activity than its pair. A great example is deltamethrin, where only one of the eight enantiomers (αS,1R,3R′-) has the desired insecticidal activity, the other seven being nonactive or less active. Only one of the enantiomers of the herbicides dichlorprop and mecoprop is responsible for their activity - the (S)-isomer is completely inactive in each case. Another in this class is haloxyfop-methyl, a chiral herbicide that was first introduced as racemate but later replaced by its R-enantiomer, responsible for the herbicidal action (10). The first study of the enantioselective biodegradation and activity of difenoconazole was carried out using a coated 5-µm tris(4-methylbenzoate) cellulosic CSP. It was used to conclude that the (2R,4S)- enantiomer would be the better choice rather than the stereoisomers to maximize bioactivity and reduce environmental damage (14). Although there have been health concerns for acute exposure to acephate related to its more toxic metabolite, methamidophos (which was banned in the European Union [EU] in 2015, although at the time of writing this article it was still in use in the US), the effect of chirality had not previously been studied. Recently, it was found that enantioselective enrichment depended on soil type and was shown to be microbially activated (15). GC–MS–MS was used, employing a heptakis(2,3-di-O-methyl-6-O-tert-butyldimethylsilyl)-β-cyclodextrin chiral GC capillary column. Sample preparation used a modified QuEChERS method, followed by drying with anhydrous magnesium sulphate to protect the chiral GC column. Relative enantioselective bioactivity of the enantiomers of acephate and methamphos is in this case, however, not so clear as it appears to depend on the species it is applied to. This study investigated the different enantioselective degradation rates under various soil conditions as a possible cause and concluded that differing microbial populations could play a significant role.
Enantiomeric Degradation
Environmental biodegradation of chiral pesticides and herbicides is frequently enantioselective. As in any guest-host interaction in biological systems, the interaction of such molecules with microorganisms in the environment is chiral and can result in differing metabolism (microbial transformation), causing possible selective accumulation of one isomer over the other. Many recent studies provide evidence of such microbial transformation by comparing transformation profiles in sterile and nonsterile soils. In the case of haloxyfop and haloxyfop methyl, a study was carried out using chiral GC–MS employing a custom made permethylated beta cyclodextrin phase (OV 1701 with 15% (w/w) permethylâβâcyclodextrin and a film thickness of 0.1 μm) (10). Haloxyfop was derivatized as the ethyl ester to enable simultaneous separation of haloxyfop and haloxyfop methyl, and the derivatization procedure was shown to be nonenantioselective. It was shown that rapid degradation by cleavage of the ester group occurred in three different types of soils studied, but was not observed in sterile soils, possibly explained by the presence of microbial carboxy esterases. Further, chiral inversion occurred, with rapid conversion of the S-enantiomer to the R-enantiomer in nonsterile soils (Figure 2), reaching a steady state when the R-enantiomer level was about 10 times that of the S-enantiomer. Interestingly, faster inversion was observed for the acid when originally applied as haloxyfop methyl.
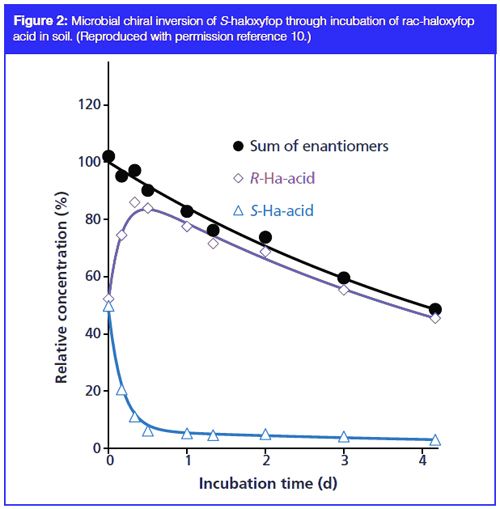
Individual enantiomers were isolated for the study using a cellulose tricinnamate CSP in 95:5:0.1 heptane–isopropanol–acetic acid, purifiying 2 mg from a total of 10 injections (20 min per injection) and confirmed with >99% enantiomeric purity by chiral GC–MS as the methyl ester. Analytically, 85:5:10 heptane–isopropanol–methanol provided a separation in under 10 min with the same column. If the herbicide is applied to the soil for root update, then this rapid interconversion to the active R-enantiomer results in independence of herbicidal activity from the enantiomeric composition applied. Any difference because of the mode of application to the growing plant was also studied, using the same GC–MS method, and found that, when applied to the leaves, no interconversion takes place such that the effect of applying individual enantiomers directly to the plant will be very different and only the R-enantiomer of haloxyfop effective (11).
A newly developed antiviral agent, dufulin, used widely in China to prevent disease in rice, tobacco, and vegetables, was found to degrade 6–8 times faster in nonsterile soils (16,17), providing confirmation of its degradation by soil microbial action but in this case without any chiral inversion. After extraction of the soil samples with acetonitrile, the chiral separation was carried out in normal phase on immobilized amylosic tris(3,5-dimethylphenylcarbamate). ECD was used to determine the absolute configurations of the two dufulin enantiomers, confirmed as the S-(+)-enantiomer for the first eluting enantiomer, and R-(−)-enantiomer as the second one.
It is also now established that there is enantioselective toxicity from many pharmaceutically active compounds and illicit drugs to freshwater species, especially through adsorption on sediments and suspended solids. For example, S-(+)-fluoxetine and S-(-)-atenolol significantly inhibit the growth of a freshwater protozoan, Tetrahymena thermophilia, compared to the opposite enantiomer (18). This study aimed to develop a comprehensive screening protocol for multiresidue identification. After microwave assisted extraction and solid-phase extraction (SPE), separations were performed on a 5-µm cellobiohydrolase CSP in reversedâphase mode for the amphetamines and, for all other analytes, on a vancomycin bonded 5-µm silica CSP (in the polar ionic mode, using methanol, 4 mM ammonium acetate and 0.005% formic acid) (see, for example, Figure 3). This method was used to investigate stereoselective effects in sludge treatment processes. In another study, microbial degradation of the chiral fungicide, benalaxyl (BX), was investigated in water, sediment, and water–sediment environments (19). A separation of the enantiomers of both the parent compound and its acid metabolite was achieved using a tris(3,5-dimethylphenylcarbamate) coated cellulosic CSP in a mobile phase of n-hexane and 2-propanol (91:9, v/v). Elution order, determined using a polarimetric detector at 426 nm, was (-)-BX, (+)-BX, (-)-BX acid, and (+)-BX acid. Sediment microbial populations were found to be responsible for enrichment of the more toxic (+)-enantiomer, causing higher risk in aquatic environments. Additionally, the (-)-enantiomer was preferentially degraded, enriching the presence of the persistent (up to 70 days) benalaxyl acid, of concern to the aquatic environment.
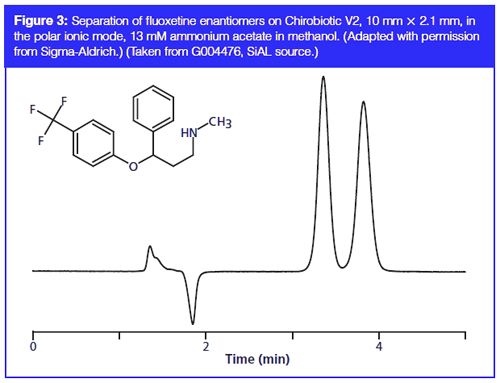
Enantioselective Transformation
A study of indoxacarb on immobilized amylosic tris(3,5-dimethylphenylcarbamate) in normal phase reported no interconversion but degradation of each isomer depended on soil pH and its microbial activity (20). Many studies have been carried out over the years on polychlorinated biphenyls (PCBs) and a recent study looked at the transfer of PCBs 95, 132, 135, and 149 into chickens via soil and chicken feed (21). The results indicated enantioselective metabolism, but nonselective maternal transfer to chicks and it was found that enantiomeric enrichment of PCBs 95, 132, and 149 and interconversion of PCB 135 later occurred in the chick resulting in different toxicity compared to the adult.
Interestingly, the unexpected appearance of the banned antibiotic chloramphenicol in animal feed has, for the first time, been traced back to its production naturally by bacterial activity in soils. Uptake into animal feed crops was studied by chiral LC–MS using an α1-acid glycoprotein CSP and found to be related to its bioavailability (22).
Summary
Stereoselective investigations need to continue to play a significant role in the study of the environmental impact of agrochemicals, POPs, and pharmaceutical products. Apart from their impact on living organisms, a critical outcome of their presence is the disruption of the natural microbial status resulting from stereospecific transformation of these molecules, as well as the potential for their enantioselective persistence in the environment. The majority of applications reported used either polysaccharide CSPs or derivatized cyclodextrin-based capillary GC columns. Although there have not been any new developments for the latter, or for protein-based CSPs, these phases retain their usefulness in this area. The advent of smaller particle CSPs for the polysaccharide CSPs has increased both speed and selectivity, enabling more complex and difficult separations to be developed, while the future of ultraefficient TPP-based CSPs bonded with a wide range of chiral selectors is set to transform chiral HPLC separations yet again.
References
- A. Cavazzini, N. Marchetti, R. Guzzinati M. Pierini, A. Ciogli, D. Kotoni, I. D’Acquarica, C. Villani, and F. Gasparrini, TrAC, Trends Anal. Chem.63, 95–103 (2014).
- F. Gritti, D.S. Bell, and G. Guiochon, J. Chromatogr. A1355, 179–192 (2014).
- O. Ismali, C. Allessia, C. Villani, and F. Gasparrini, J. Chrom. A1427, 55–68 (2016).
- D.C. Patel, Z.S. Breitbach, M.F. Wahab, C.L. Barhate, and D.W. Armstrong, Anal. Chem.87, 9137–9148 (2015).
- C.L. Barhate, M.F. Wahab, Z.S. Breitbach, D.S. Bell, and D.W. Armstrong, Anal. Chim. Acta898, 128–137 (2015).
- X. Zhang, F. Luo, Z. Loum, and M. Lu, J. Chrom. A1359, 212–223 (2014).
- Q. Fu, Y. Wang, J. Zhang, H. Zhang, C. Bai, J. Li, W. Wang, H. Wang, Q. Ye, and Z. Li, J. Agric. Food Chem.61(32), 7689–7695 (2013).
- Y. Tao, F. Dong, J. Xu, X. Liu, Y. Cheng, N. Liu, Z. Chen, and Y. Zheng, J. Agric. Food Chem.62(47), 11457–11464 (2014).
- Q. Zhang, M. Tian, M. Wang, H. Shi, and M. Wang, J. Agric. Food Chem.62(13), 2809–2815 (2014).
- T. Poiger, M.D. Müller, H.-R. Buser, and I.J. Buerge, J. Agric. Food Chem.63(10), 2583–2590 (2015).
- I.J. Buerge, A. Bächli, W.E. Heller, M. Keller, and T. Poiger, J. Agric. Food Chem.63(10), 2591–2596 (2015).
- Y. Huang, Y. Xu, J. Li, W. Xu, G. Zhang, Z. Cheng, J. Liu, Y. Wang, and C. Tian, Environ. Sci. Technol.47(23), 13395–13403 (2013).
- C. Caballo, M.D. Sicilia, and S. Rubio, Talanta134, 325–332 (2015).
- F. Dong, J. Li, B. Chankvetadze, Y. Cheng, J. Xu, X. Liu, Y. Li, X. Chen, C. Bertucci, D. Tedesco, R. Zanasi, and Y. Zheng, Environ. Sci. Technol.47(7), 3386–3394 (2013).
- X. Wang, Z. Li, H. Zhang, J. Xu, P. Qi, H. Xu, Q. Wang, and X. Wang, Environ. Sci. Technol.47(16), 9233–9240 (2013).
- H.Z. Wang, H.G. Zuo, Y.J. Ding, C. Jiang, and H. Zang, Environ. Sci. Pollut. Res. Int.6, 4331–42 (2014).
- K.-K. Zhang, D.-Y. Hu, H.-J. Zhu, J.-C. Yang, and B.-A. Song, J. Agric. Food Chem.62(8), 1771–1776 (2014).
- S.E. Evans, P. Davies, A. Lubben, and B. Kasprzyk-Hordern, Anal. Chim. Acta882, 112–126 (2015).
- M. Liu, D. Liu, Y. Xu, X. Jing, Z. Zhou, and P. Wang, J. Agric. Food Chem.63(21), 5205–5211 (2015).
- Y.-P. Zhang, D.-Y. Hu, H.-R. Ling, L. Zhong, A.-X. Huang, K.-K. Zhang, and B.-A. Song, J. Agric. Food Chem.62(37), 9066–907 (2014).
- X.-B. Zheng, X.-J. Luo, Y.-H. Zeng, J.-P. Wu, and B.-X. Mai, Environ. Sci. Technol. 49(2), 785–791 (2015).
- B. Berendsen, M. Pikkemaat, P. Römkens, R. Wegh, M. van Sisseren, L. Stolker, and M. Nielen, J. Agric. Food Chem.61(17), 4004–4010 (2013).
Denise Wallworth is with SigmaâAldrich UK, a subsidiary of Merck, in Poole, Dorset, UK. Direct correspondence to: denise.wallworth@sial.com

Determining Enhanced Sensitivity to Odors due to Anxiety-Associated Chemosignals with GC
May 8th 2025Based on their hypothesis that smelling anxiety chemosignals can, like visual anxiety induction, lead to an increase in odor sensitivity, a joint study between the University of Erlangen-Nuremberg (Erlangen, Germany) and the Fraunhofer Institute for Process Engineering and Packaging (Freising, Germany) combined behavioral experiments, odor profile analysis by a trained panel, and instrumental analysis of odorants (gas chromatography-olfactometry) and volatiles (gas chromatography-mass spectrometry).
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










