HPLC Column Technology in a Bioanalytical Contract Research Organization
Special Issues
When selecting the optimum phase for SEC separations, several key column parameters must be considered carefully.
Ryan Collins and Shane Needham, Alturus Analytics, Inc., Moscow, Idaho, USA.
High performance liquid chromatography–tandem mass spectrometry (HPLC–MS–MS) is the go-to technique for high-throughput analysis of small-molecule therapeutics, metabolites, and biomarkers. Through technological advancements in the last decade, developing quality methods for a novel analyte in the contract research environment has become easier and faster than ever. Increasingly shorter run times, higher sensitivity, and greater separation have all become possible in a standard method. This is, in part, because of column technologies that have enabled the standardization of the method development process. Method efficiency and productivity are also improving because of emerging column technologies such as sub-2-µm particles coupled with ultrahigh-pressure liquid chromatography (UHPLC)–MS–MS, superficially porous particle columns, and microflow HPLC–MS–MS. Increasing efficiency and productivity in high-throughput bioanalysis is becoming more important as the applications for HPLC–MS–MS expand to large molecules such as peptides, proteins, and oligonucleotides.
Over the course of the last several decades, high performance liquid chromatography–tandem mass spectrometry (HPLC–MS–MS) has become the method of choice for high-throughput analysis of smallâmolecule therapeutics, metabolites, and biomarkers. This is due, in large part, to the selectivity and sensitivity provided by HPLC–MS–MS, combined with the ability to rapidly develop an assay consisting of quick extractions and short run times for a vast majority of small molecules.
When presented with a new analyte at a bioanalytical contract research organization (CRO), the goal is to develop a robust method with good chromatographic resolution, repeatable results, and a quick run time. However, after these scientific criteria have been met, the ultimate end goal for any bioanalytical CRO is productivity and efficiency - analyzing the most samples possible while using the minimum amount of solvent, supplies, and resources, and still remaining scientifically sound. In short, the goal at a CRO is to create the most productive method in the most efficient manner possible, all while using sound science. This approach benefits not only the CRO, but also its bioanalytical clients, and most importantly, the end users; patients that can receive care from these novel therapeutics provided by the industry.
There is an increasing trend in the industry to monitor (possibly multiple) metabolites, as well as a push towards using HPLC–MS–MS for the analysis of large molecules, including peptides and proteins. As the industry shifts towards the analysis of more-complicated therapeutics, there is a need to increase efficiency and productivity wherever possible. With that in mind, when developing a new HPLC–MS–MS method for a novel molecule, every tool in the chromatographic arsenal should be used to grant the best chance of success. Perhaps the strongest, most versatile tool in the bioanalytical setting is the LC column. A large reason that method development can be performed with the amount of efficiency necessary to function as a CRO in today’s bioanalytical world is the development of column technology over the past few decades. The reliable repeatability of columns on the market today, combined with the plethora of unique column types that can be implemented, allow for the efficient development of an HPLC–MS–MS method for high-throughput analysis.
Because all bioanalytical work depends on high-throughput analysis, many of the trends in emerging technologies in the bioanalytical market are directly related to increasing on-instrument productivity and reducing costs. This includes smaller particle size in columns coupled with ultrahigh pressure liquid chromatography (UHPLC), superficially porous shell column technology, and microflow HPLC. This article presents a quick background into the details of developing an HPLC–MS–MS method from the perspective of a CRO in relation to column choice. It also focuses on recent column technologies, the instrumentation surrounding them, and their benefits in a CRO environment.
Method Development
High-throughput bioanalysis CROs are usually a fast-paced environment, where it is necessary to create a productive, rugged method from the ground up for what is often times an unknown novel therapeutic. A large part of a CRO’s efficiency stems from its ability to quickly develop a rugged method that will repeatedly hold up to rigorous industry and regulatory standards. As efficiency can often be derived from simplicity, when developing a new method the simplest solution is always the first approach. This is why, despite the plethora of columns available for use, it is almost always best to start with a C18 or C8 column. One of the most versatile and widely used columns, the C18 column has been in use in one form or another for decades. Comprising a simple octadecyl carbon chain bonded silica-based stationary phase, the C18 column is the go-to column of choice for a large majority of molecules analyzed by HPLC–MS–MS. C18 columns have proven to provide good retention and resolution for a vast array of small molecules.
With a proven track record of negligible lot-to-lot and columnâtoâcolumn variability, there is minimal concern of anomalous behaviour throughout the life of a method on a C18. C18 columns also tend to be very rugged, with the average lifespan lasting for upwards of thousands of injections. This is a very important point in the development of any method; if a seemingly scientifically sound method has been developed, but the column only lasts a few hundred injections before peak deterioration, then the method probably isn’t rugged or productive enough to be feasible. A large benefit in the flexibility of the C18 is that it allows for the standardization of many HPLC–MS–MS methods, which greatly increases the productivity of high-throughput analysis. With multiple standardized methods relying on one type of column and identical mobile phases for an array of molecules, it is possible to keep instruments running continuously without interruption. This is crucial to the high-volume requirement in the bioanalytical CRO world.
However, there are always going to be analytes that do not work on a C18 column. For multiple analytes, resolution (Rs) and chromatographic selectivity (α) will play a role. However, here we focus on method development of one analyte. Whether due to poor retention (tR), poor asymmetry factor (AF), or poor repeatability, decisions can then be made on what type of specialty column to look at. This process can quickly become overwhelming given the plethora of columns and column types on the market today. Having an approach to address the most common column-based issues during method development, as seen in the flowchart in Figure 1, is an important aspect in maintaining efficiency during method development. Once it has become apparent that a method will not be adequately developed on a C18 column, the next step is typically to evaluate the polar moieties and functional groups exhibited by the molecule. For a polar molecule, some of the more common approaches available are to choose a polar endcapped column or to implement an ion-pairing reagent (where an ion-pairing reagent such as heptafluorobutyric acid [HFBA] is added to the mobile phases or extraction solvents). When presented with a particularly small, polar molecule, another option available is to choose a column such as an F5 column (a pentafluorophenylpropyl stationary phase) or to use a hydrophilic-interaction chromatography (HILIC) method. HILIC methods use gradients with a high percentage of organic content coupled with either an unmodified silica column, an amino column, a zwitterionic column, or any one of a number of columns made specifically for HILIC methods.
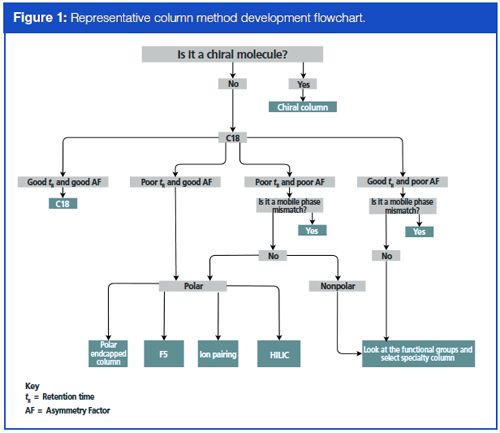
Recent Column Advancements
Although efficiency in method development is paramount to being cost effective in a bioanalytical CRO environment, this efficiency would amount to nothing if the actual methods themselves were not productive in the long run. Even if all the scientific benchmarks may have been met during development, the overall costs of performing the method determine whether it will actually be feasible. The costs of a method are largely determined based on two factors: the overall costs of disposable supplies (for example, extraction supplies, solvents, and columns) and time. With this in mind, it is no surprise that many of the emerging technologies in the industry seek to minimize both of these aspects of HPLC–MS–MS.
One such way to increase HPLC–MS–MS productivity that has been developed and implemented in the past decade is the decrease in column packing particle size. Traditionally, the packing in LC columns has been made up of fully porous particles ranging in size from 3 to 10 µm. However, by decreasing the particle size below the previous standards to sub-2âµm particles, there is an increase in chromatographic efficiency leading to an increase in theoretical plate counts and, thus, greater resolution (1). However, one of the side effects of decreasing the particle size is a fairly large increase in pressure, which limited the widespread use and commercial viability of subâ2âµm columns until fairly recently. To withstand the back pressures involved with using subâ2âµm columns, new instrumentation was devised; thus, UHPLC was born (2,3). Using an UHPLC system available from various vendors, it is possible to successfully implement smaller particle size columns and run at pressures as high as ~20,000 psi (4). These UHPLC systems have proven to be robust enough for highâthroughput bioanalysis work and have been implemented throughout the industry.
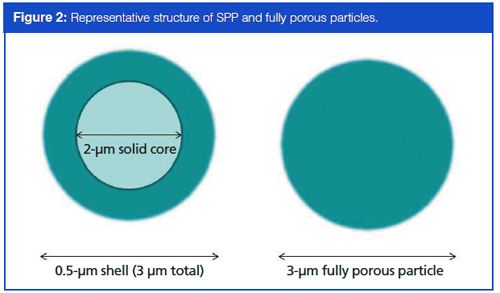
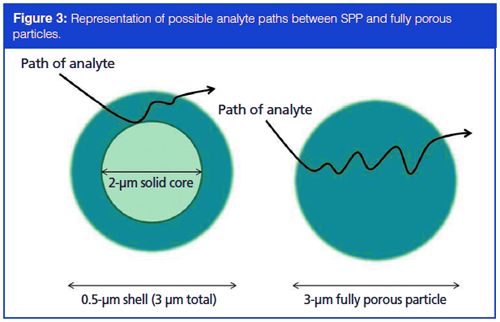
However, since cost effectiveness is an overall goal of a bioanalytical CRO, it may not be the most practical option to purchase an entirely new HPLC system to attain what may amount to only a slight increase in method productivity and decrease in run time. For laboratories already in existence and set up with traditional HPLC instruments rather than UHPLC, it is much more desirable to find a smaller-scale solution to increase method productivity. Another recent advent to the column market in the last decade, superficially porous particle (SPP) columns take the idea of smaller column particles to the next, albeit somewhat divergent, step. Rather than decreasing the size of fully porous particles in the columns, the idea behind SPP is a small, solid inner core (which generally range from 1.3 to 5 µm) surrounded by a permeable shell of porous silica. While the outer shell of the particles are similar in materials and function as a conventional fully porous column particle, the inner core is impermeable (hence the term superficially porous), as can be seen in Figure 2. Although the idea of shell-based stationary phases have been around since the late 1960s, with the use of larger (~50 µm) pellicular particles (5), it is only recently that the particle sizes have been reduced down to conventional standards. With the combination of the small diameter of the inner core and the porous nature of the shell, SPPs provide the benefits of sub-2-µm fully porous particles while eliminating many of the back pressure issues (6,7). Although the theory behind the increase in efficiency attributed to SPP columns is not discussed in detail here, Figure 3 shows a rough representation of how the rate of diffusion is increased throughout an SPP column as opposed to a column containing fully porous particles of comparable size. This increased rate of diffusion relates to quicker, more efficient separations than were previously possible with fully porous columns, and results in a tighter peak shape as the shorter path reduces the diffusion of the analytes (8).
Emerging Technologies
Yet another approach of reducing costs and increasing efficiency in bioanalytical analysis is the implementation of microflow LC coupled with a mass spectrometer. As compared to the standard high flow of HPLC–MS–MS, which generally uses around a 700-µL/min flow rate, microflow LC–MS–MS employs the use of pumps that can accurately deliver a flow rate of well below 100 µL/min, greatly reducing the consumption of solvents. This reduction in solvent use directly translates to a cost savings in the purchasing of solvents, disposal of solvent waste, and the labour of solvent preparation - none of which are insignificant expenses in a highâthroughput laboratory that is virtually running continuously. The drastically lower flow rates associated with microflow LC–MS–MS also translate to less solvent flowing through the electrospray ionization (ESI) source. This means a cleaner MS system and a lower cost associated with MS maintenance.
Microflow LC–MS–MS employs columns with drastically decreased internal column diameter. While standard HPLC–MS–MS may use columns with internal diameters ranging from 2 to 4.6 mm, microflow LC–MS–MS uses columns with internal diameters ranging from 0.2 to 0.3 mm (micro) down to <0.2 mm (nano), which can be used with flow rates of 10 and 0.3 µL/min, respectively. Solvent consumption and savings aside, microflow LC–MS–MS has also been documented to increase ESI response (9) while reducing matrix effects (10) and increasing ionization efficiency (11). Early works on ESI response demonstrated that as the mobileâphase flow rate of ESI is reduced, there is an increase in proportional MS signal-to-noise ratio (12).
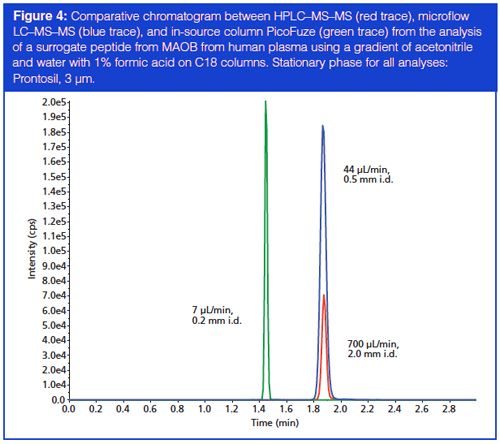
Some of the challenges in the integration of microflow LC–MS–MS into the high-throughput bioanalysis world are longer run times, dead volumes in fittings and connections having a greater impact on chromatography, and a perceived lack of robustness of microflow instrumentation. To address some of these challenges, work has been performed by multiple vendors on implementing an integrated, in-source column. By integrating a column directly into the source, many of the dead volume issues related to microflow LC–MS–MS are resolved. The idea behind the application is to simplify instrument setup by minimizing connections and reducing the length of tubing required between the LC injector and the MS, and thus minimizing the impact of pre-column and post-column volumes. As shown in the corresponding chromatogram in Figure 4, the combination of a micro internal diameter column integrated into the source coupled with microflow LC–MS–MS provides a greatly increased signal as compared to HPLC–MS–MS; in addition, the system maintains a run time of less than 5 min. With the possibility of a system that is generating higher sensitivity (among other chromatographic benefits) coupled with lower flow rates leading to lower solvent consumption, microflow LC–MS–MS combined with integrated, in-source columns seems to be a highly promising direction for high-throughput bioanalysis.
Conclusion
With the advancements in column and other LC technology in recent years, developing robust methods for novel therapeutics has become a more reliable process than ever. It is possible to efficiently create productive methods for molecules of ever-increasing complexity. This will become more important in years to come as HPLC–MS–MS is increasingly looked to as the solution for analysis of large molecules including peptides, proteins, and biomarkers. Increasing efficiency and productivity on both the front end (method development) and back end (sample analysis) will be made continuously possible with further advancements such as SPP columns and microflow LC–MS–MS. Looking to the future, the expectation for the pharmaceutical and biotech industries will be to supply the global community with therapeutics at a reasonable cost. Thus, the highest levels of productivity and efficiency will be paramount to meet this goal.
References
- J.E. MacNair, K.C. Lewis, and J.W. Jorgenson, Anal. Chem.69, 983–989 (1997).
- J.E. MacNair, K.D. Patel, and J.W. Jorgenson, Anal. Chem.71, 700–708 (1999).
- N. Wu, J.A. Lippert, and M.L. Lee, J. Chromatogr. A911, 1–12 (2001).
- “In the News”, Trends Anal. Chem.61, iv–x (2014).
- C. Horváth, B.A. Preiss, and S.R. Lipsky, Anal. Chem.39, 1422 (1967).
- J.J. DeStefano, T.J. Langlois, and J.J Kirkland, J. Chrom. Sci.46, 254–260 (2008).
- D.V. McCalley, J. Chromatogr. A1218, 2887−2897 (2011).
- G. Guiochon and F. Gritti, J. Chromatogr. A1218, 1915–1938 (2011).
- G. Valaskovic and N. Kelleher, Curr. Top. Med. Chem. 2(1), 1–12 (2002).
- E. Gang, M. Annan, N. Spooner, and P. Vouros, Anal. Chem.73(23), 5635–5644 (2001).
- R. Juraschek, T. Dulcks, and M. Karas, J. Am. Soc. Mass Spectrom.10, 300–308 (1999).
- P. Kebarle and L. Tang, Anal. Chem.65, 972A–986A (1993).
Ryan Collins and Shane Needham are with Alturus Analytics, Inc., in Moscow, Idaho, USA. Direct correspondence to: sneedham@alturasanalytics.com

University of Oklahoma and UC Davis Researchers Probe Lipidomic Profiles with RP-LC–HRMS/MS
May 6th 2025A joint study between the University of Oklahoma Health Sciences Center (Oklahoma City, Oklahoma) and the UC Davis West Coast Metabolomics Center (Davis, California) identified differentially regulated lipids in type 2 diabetes (T2D) and obesity through the application of reversed-phase liquid chromatography-accurate mass tandem mass spectrometry (RP-LC-accurate MS/MS).
Altering Capillary Gas Chromatography Systems Using Silicon Pneumatic Microvalves
May 5th 2025Many multi-column gas chromatography systems use two-position multi-port switching valves, which can suffer from delays in valve switching. Shimadzu researchers aimed to create a new sampling and switching module for these systems.
Studying Cyclodextrins with UHPLC-MS/MS
May 5th 2025Saba Aslani from the University of Texas at Arlington spoke to LCGC International about a collaborative project with Northwestern University, the University of Hong Kong, and BioTools, Inc., investigating mirror-image cyclodextrins using ultra-high performance liquid chromatography–tandem mass spectrometry (UHPLC–MS/MS) and vibrational circular dichroism (VCD).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










