Perspectives in Hydrophobic Interaction Temperature-Responsive Liquid Chromatography
Temperature-responsive liquid chromatography (TRLC) is an emerging green high performance liquid chromatography (HPLC) mode allowing reversed-phase-type separations while necessitating only water as the mobile phase. The columns therein are typically packed with silica particles to which stimuli-responsive polymers are anchored. In hydrophobic interaction TRLC, such polymers depict a loss of water solubility when increasing the temperature above a characteristic conversion temperature, causing large changes in retention over quite narrow and mild temperature ranges (~5–55 °C). TRLC circumvents the concerns about analyte or column degradation that can occur when implementing high temperatures (>80 °C) on conventional reversed-phase columns. It allows for HPLC using only water often spiked with the additives typically used in reversed-phase LC. Therefore, this separation mode allows for greener, cheaper, and isocratic analyses under non-denaturing conditions. The absence of compositional solvent gradients also allows for the exploitation of temperature gradients in combination with refractive index detection. Purely aqueous hydrophobic interaction TRLC is mostly applicable for solutes depicting a 1 < LogP < 5, yet these ranges can be expanded through implementation of combined aqueous or organic mobiles phases, while preserving the temperature-responsive effects. In this first TRLC instalment, our recent developments, new possibilities, and current limitations of the use of 1D TRLC are discussed, while the column performance is described with respect to the fundamentals of HPLC.
Temperature-responsive liquid chromatography (TRLC) is an emerging separation mode in liquid chromatography that makes it possible to obtain reversed‑phase-type separations under purely aqueous conditions. In this mode, retention is controlled through the column temperature while the mobile phase is invariable and composed of only water. This stands in contrast with conventional reversed-phase liquid chromatography (LC) performed on, for example, octadecyl silica (C18), whereby compositional solvent gradients are usually necessary to allow timely elution of disparate molecules in terms of polarity and retention. Therefore, TRLC avoids the need for organic cosolvents in the aqueous mobile phase.
The approach has mainly been based on the exploitation of a particular class of stimuli-responsive (often dubbed “smart”) polymers that lose their water solubility once a certain temperature is exceeded. The temperature at which this transition occurs depends mainly on a lower critical solution temperature (LCST), which is characteristic for each polymer. However, the molecular weight, concentration, and (poly)dispersity also influence the experimental polymer precipitation temperature. In TRLC, the polymer chains are typically anchored to silica particles and packed into stainless steel high performance liquid chromatography (HPLC) columns. The concept was introduced by Kanazawa and associates in the early 1990s, followed by a variety of demonstrations and enhancements, including by our group, in the last two decades (1–8).
Temperature-responsive polymers depict a drastic change in their physical properties with temperature. In general, such behaviour in polymers is not uncommon, providing one can consider any solvent composition. Given that only a limited number of polymers are responsive in water and around physiological temperatures, these polymers have attracted the most attention for applications such as cell culture release and drug delivery, and for their potential in LC (9,10). This phenomenon has mostly been studied in substituted polyacrylamides, with particular emphasis on poly(N-isopropylacrylamide) (PNIPAAm). The utilization of the ensuing stationary phases in LC makes it possible to obtain increased or reduced retention at higher or lower temperatures, respectively, as illustrated in Figure 1. This is caused by the formation of an apolar layer on the surface of the stationary phase owing to the precipitation (demixing) of the polymer from the aqueous mobile phase at higher temperatures. Correspondingly, cooling down the column resolubilizes the polymer chains, concomitantly eliminating their retentive ability. The retention mechanism is based on hydrophobicity and is therefore similar to that of reversed-phase LC. However, it allows operation with only water as the mobile phase, whereby the retention increases drastically with mild increases in temperatures. In this article, the possibilities and limitations of TRLC are discussed with respect to chromatographic fundamentals.
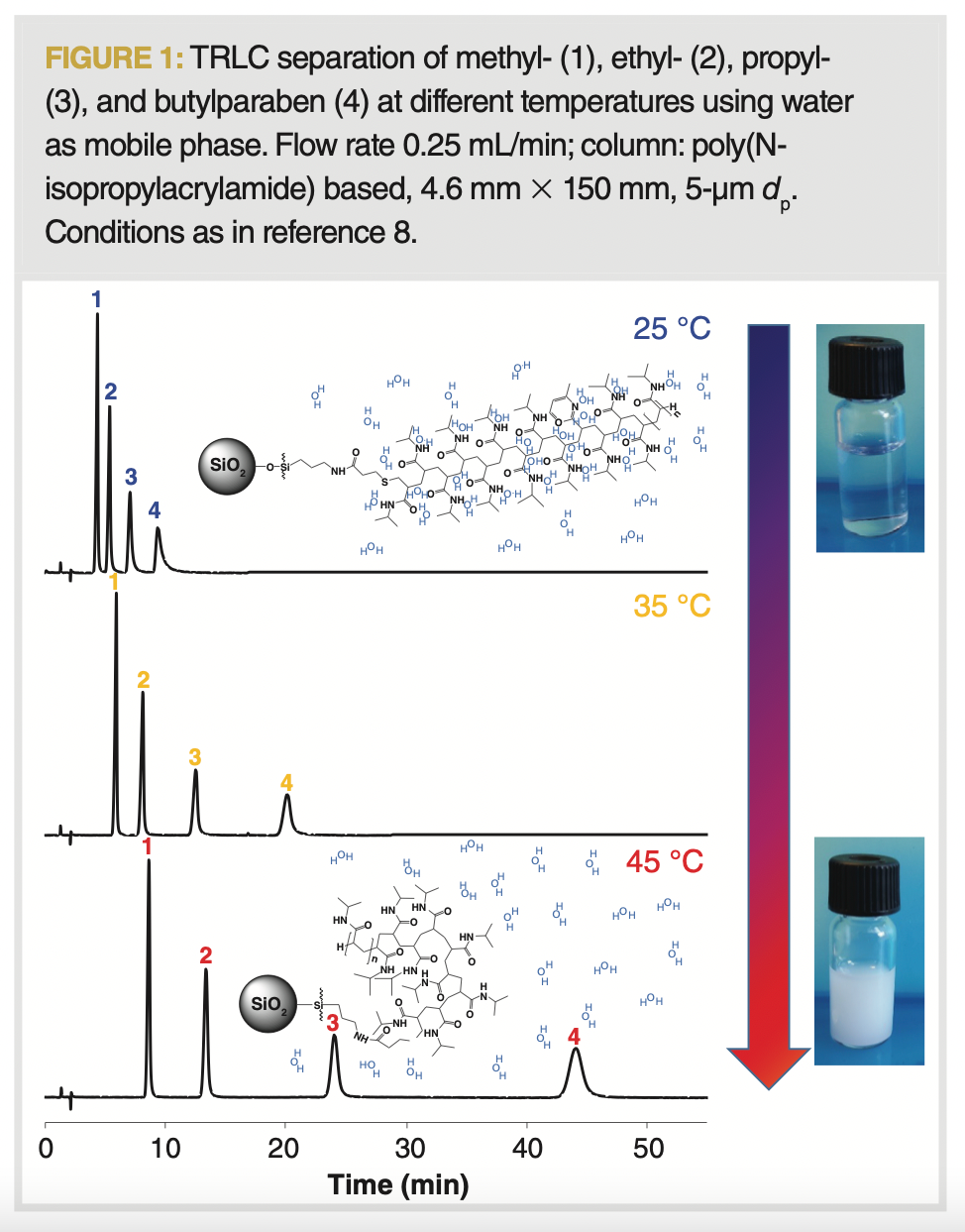
Retention
Thermodynamics of Retention: In reversed-phase LC, one typically observes a decrease in retention when increasing the temperature. Thermodynamically, this means that retention is enthalpically (∆Hretention) favourable, and entropically (∆Sretention) unfavourable (11). As chromatographic retention is usually exothermic, the Gibbs free energy equation (∆Gretention = ∆Hretention – T∆Sretention) shows that when increasing the temperature, the retention phenomenon becomes less favourable (as ∆Gretention is becoming less negative at elevated temperatures). This can be represented via a Van ‘t Hoff plot (ln k vs 1/T), as shown, for example, for Linuron in Figure 2, whereby it can be seen that indeed the retention is slowly decreasing with increasing temperature on a C8 column (12).
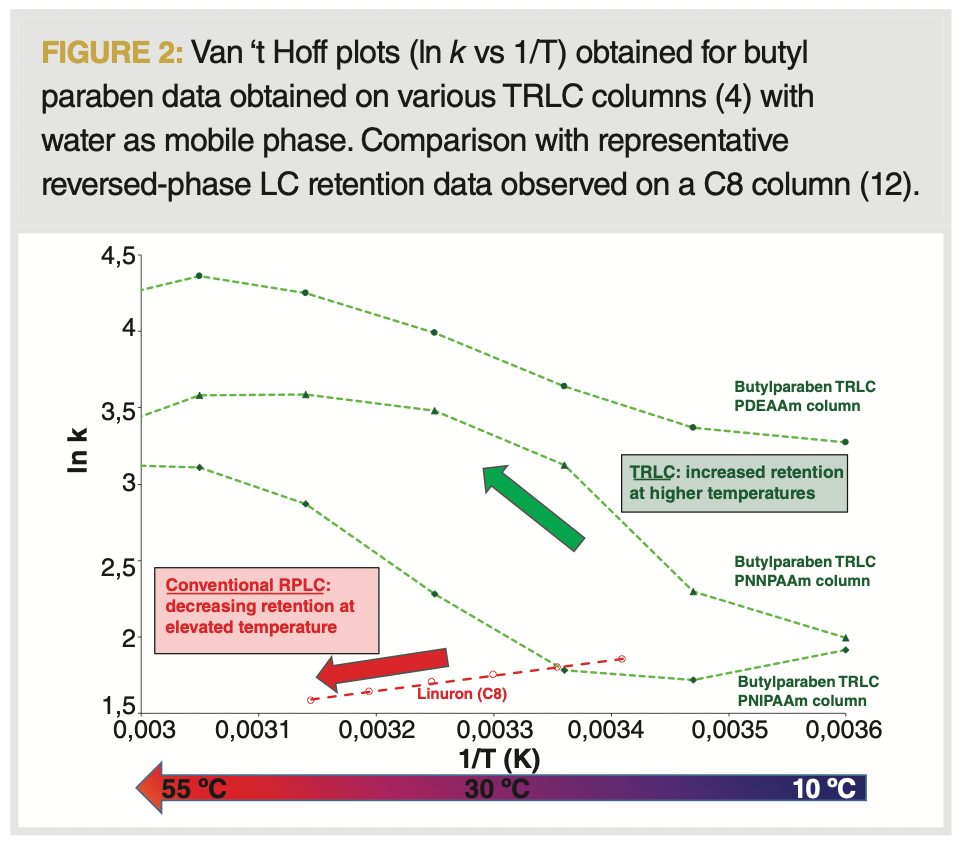
In TRLC, this retention behaviour is drastically altered because the stationary phase itself (and hence mainly ∆Hretention) undergoes a radical, reversible change from a dissolved, highly hydrated random coil to a more condensed globular shape. Thermodynamically, this conversion is entropically driven. The enthalpy contribution of dissolving the polymer in water is exothermic (∆Hpolymer dissolution <0) because of the formation of favourable hydrogen bonds between the water molecules and the polar groups of the polymer, leading to hydration of the polymer. However, dissolving macromolecules with apolar groups on the backbone and side-chain causes a significant loss in entropy (∆Spolymer dissolution <0), as the water molecules are forced into a cage-like orientation around these hydrophobic parts of the polymer (see Figure 1). As the temperature increases, the enthalpy contribution becomes weaker and the entropic loss becomes more pronounced, leading to a positive Gibbs free energy (∆Gpolymer dissolution = ∆Hpolymer dissolution – T∆Spolymer dissolution), which causes the dehydration of the polymer chains. Before the dehydration, the hydrophobic side chains on the polymer are spread over a larger volume providing little retention. After the dehydration, the polymer exposes dense hydrophobic zones, allowing for enlarged retention based on hydrophobicity.
It is interesting to observe that, when the polymer is fully hydrated under cold conditions or fully precipitated in a warm environment, further temperature change beyond these points causes the normal thermodynamic behaviour to take (back) the upper hand. This is also visible through the chromatograms represented in Figure 3 obtained for a number of TRLC columns based on various polymers.
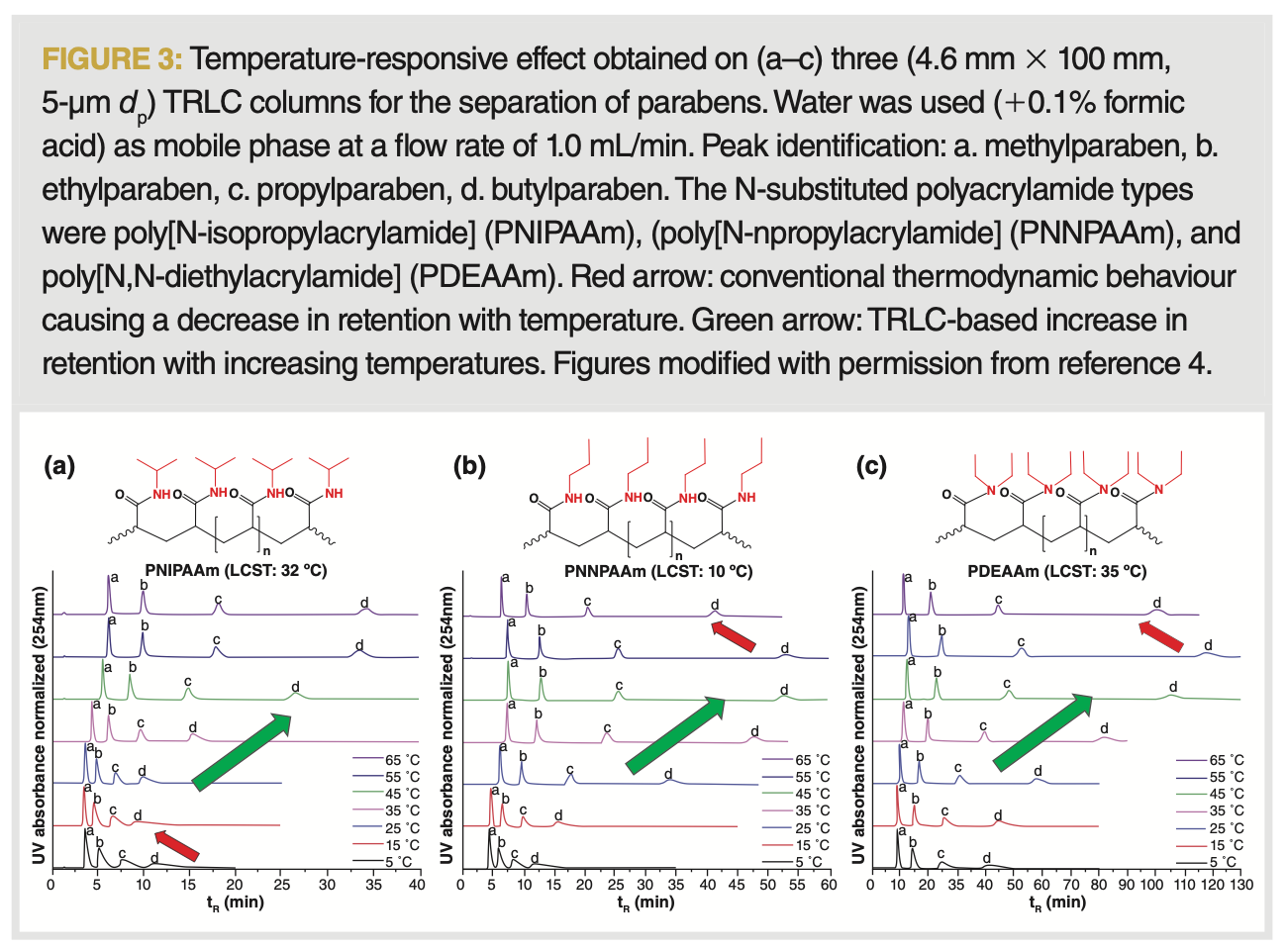
Other Aspects Controlling Retention in TRLC: Chromatographically, one aims for retention factors (k) >3 and <10, as this maximizes resolution while avoiding excessive analysis times. As illustrated in Figure 2 and 3, the use of different polymers (on otherwise identical columns) leads to different retention time changes as a function of temperature. When the isopropyl group in the most used PNIPAAm columns is altered for a linear n-propyl chain, the conversion temperature in the resulting PNNPAAm column drops to lower temperatures, while larger retention is obtained for the parabens compared to the first column type. From an HPLC point of view, the ability to perform TRLC at even lower temperatures could become useful because of the related diminished potential issues in terms of analyte stability and silica hydrolysis. When the single propyl groups are replaced by two ethyl groups per monomer, a more strongly retentive column is obtained, which might offer promise for applications where the PNIPAAm columns offer too little retention (such as for biomolecule analyses). A current limitation of TRLC is that, even at the lowest retentive conditions on all TRLC columns, a residual retention is obtained. Research has also been performed based on the implementation of co-polymers, whereby in most cases one of the monomers depicted TRLC behaviour and the other allowed for enhanced hydrophobic or coulombic retention. While copolymers can allow for better biomolecule retention, it also often further increases the residual column retention while reducing the range of retention factors that can be effectively covered through TRLC (1). As the latter is already currently not large in TRLC (see Figure 3[a], where k varies from 7 to 23 on a PNIPAAm column for butylparaben), the incorporation of sections in the polymer that do not depict temperature responsive behaviour diminishes the range of retention covered with a column. Conversely, the combination of two or more temperature-responsive polymers in a column might be promising for being able to exploit a broader reachable retention time window. Various studies have also reported the use of cross-linked networks, as compared to linear polymer strands. While promising applications have been shown, this does not seem to offer the prospect of generically applicable columns, as they offer a more limited span of reachable retention factors (9).
Next to temperature and polymer composition, the retention also proves dependent on the grafting density of the polymer brushes immobilized on the silica particles, the molecular weight, the polydispersity, the coupling chemistry, and on surface characteristics of the supporting material. While these aspects can be controlled, an evolution towards narrower polymer dispersity immobilized on a highly controlled surface topology will allow further incremental improvement of TRLC. Most examples of successful TRLC indicate a carbon load on the silica > 15%, whereby polymers depict between 40 and 50 repeating units with as narrow a distribution as possible. While obtaining a shift in retention with temperature is essential in TRLC, the magnitude of this shift can vary from a modest doubling of retention to a sometimes about 10‑fold increase. Ideally, one would strive in TRLC to obtain a similar (extremely broad) ability to tune k from 0 to about infinite, as is the case when using different solvent compositions in reversed-phase LC. While this is currently unachievable, the ability to control k even over a comparatively narrow order of magnitude already offers significant benefits in terms of gradient peak focusing and analysis time reductions (13,14).
Unsurprisingly, the composition of the mobile phase also plays a strong role in the conversion of the polymer. The addition of salts favours the dehydration of polymers, which lowers the dehydration temperatures, and consequently enhances the column retention at a given temperature (8). A drawback thereof is that in order to obtain a useful influence, high (0.2–2 M) concentrations are required. While volatile salts can also be used, this approach is nevertheless demanding for the instrumentation and columns. Alternatively, organic modifiers also strongly influence the polymer conversion temperature and the overall retention (3,7). While they strongly decrease the retention, the addition of relatively small fractions (5–15%) can be beneficial, as it can remove (or reduce) the residual polymer retention at lower temperatures and offer enhanced efficiencies, as well as elution of highly hydrophobic solutes that cannot be eluted with only water as the mobile phase. Although this approach is contrary to the green incentive to develop TRLC, it might offer promise when combined with biosourced or biodegradable solvents.
Efficiency
In general, polymer-based columns are often perceived to offer only a limited column efficiency as compared to silica-based columns (15). Because, in TRLC, rather short polymer strands are immobilized on silica, similar column efficiencies and plate heights can be expected to be reachable as obtained with reversed-phase LC columns. The best reported performances (for well retained solutes) in TRLC offer column efficiencies that are only 33% below the optimal performance reachable on C18 columns. Correspondingly, the minimal reduced plate heights (h = H/dp) are close to three (instead of two as obtained on a column packed with fully porous C18 particles). The van Deemter plots obtained for the three TRLC columns are represented (Figure 4[a]) as collected under the most retentive conditions (55 °C), and compared with an earlier TRLC study (described in Figure 4[b]) where a different (free instead of a controlled radical polymerization) synthetic route was followed.
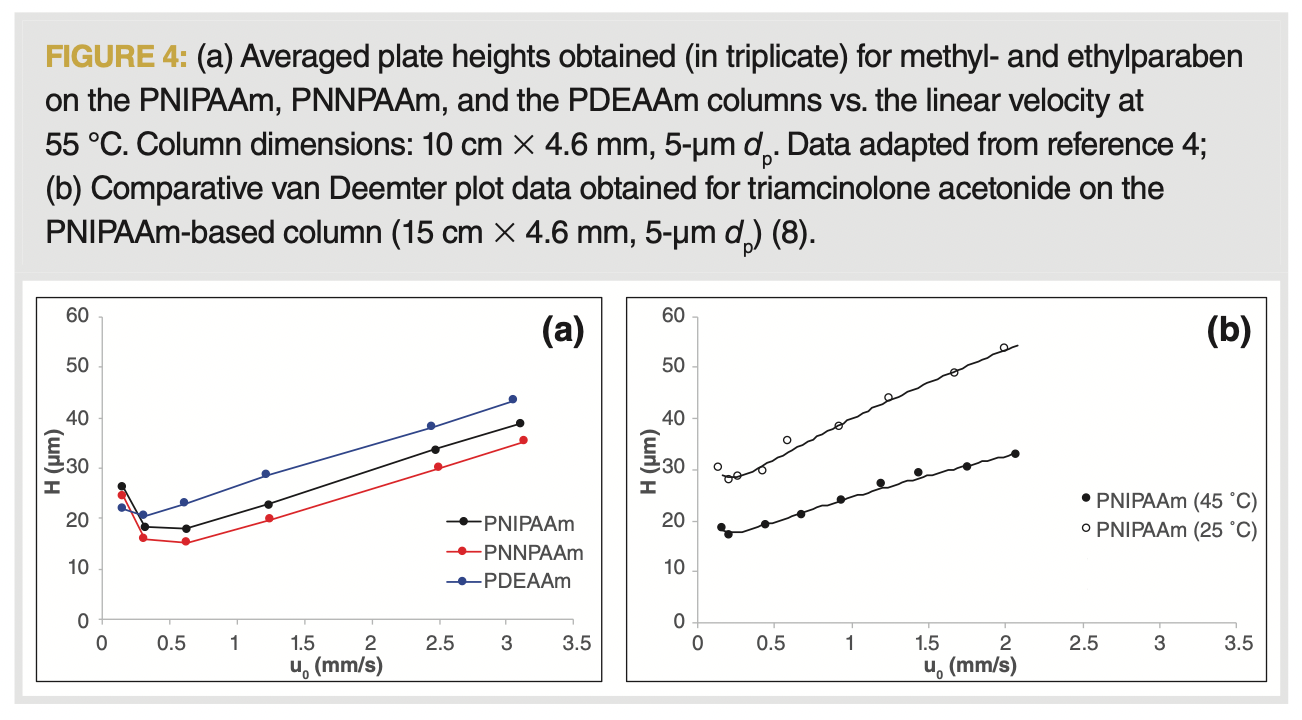
While this illustrates that indeed reasonable and improvable efficiencies can be obtained in TRLC, it can also be seen that H/u plots are characterized by a rather steep C-term regime. This is attributed to the resistance to mass transfer in the stationary phase (Cs), which is inevitably larger in a bulky polymer layer as compared to a thin layer of C18 groups. As a consequence, the optimal linear velocity is found at about 3–4 times slower flow rates compared to conventional reversed-phase LC. Hence, to reach optimal chromatography in TRLC, longer analysis times are still currently a necessity. Without a doubt, TRLC can become more efficient through the use of smaller fully or partially porous particles. The main hurdle therein has been the current practical unviability of sub-2-µm silica-based supporting materials that offer a suitable target functional group to which the polymers can be covalently anchored. In most TRLC work, the polymers are synthesized such as to contain a carboxylic end group, which can then be attached to aminopropyl silica via various amide bond forming chemistries (2,4,6,8).
On the other hand, when TRLC is performed under the poorly retentive low temperature conditions, the polymer layer is extended, and therefore more voluminous. This detrimentally affects the mass transfer (as shown in Figure 4[b] at 25 °C for PNIPAAm) and the corresponding efficiencies obtained under those conditions. However, this is unproblematic in terms of the applications, as in most cases a downwards temperature gradient is used, causing the peaks to refocus (as explained later in the section “Gradient Analyses”).
Selectivity
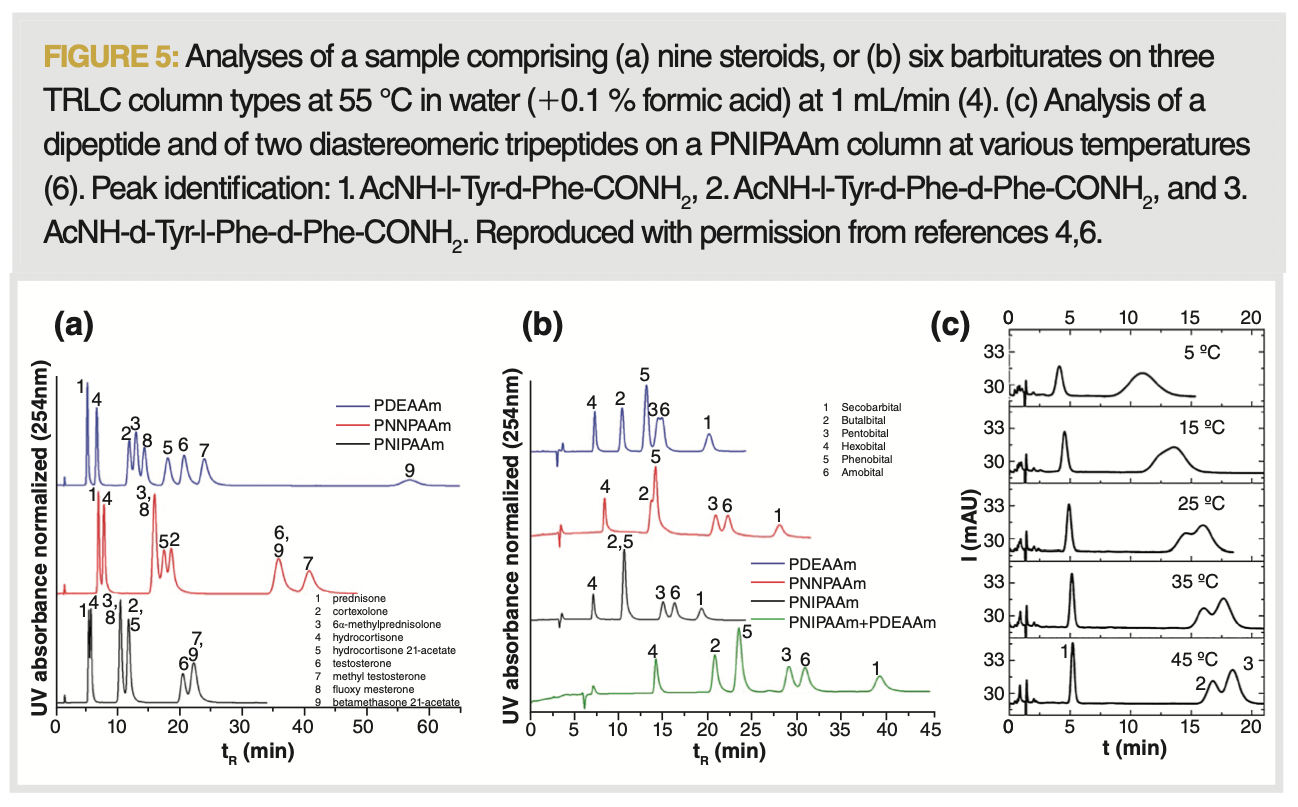
The selectivity in TRLC is altered through temperature, polymer composition, and additives. While at lower temperatures some enhanced retention of polar solutes can be observed, this has not led to promising applications. This is, in part, related to the aforementioned poor efficiencies obtained at low temperatures. At temperatures above the LCST, the retention is mainly based on hydrophobicity. When altering the polymer composition, different selectivities are observed, even for the three conceptually similar polyacrylamide polymers. This is illustrated in Figure 5, where changes in elution order are apparent for the analysis of steroids and barbiturates. The different selectivities can also be combined via a stationary‑phase optimized selectivity LC-type of approach to deliver an optimized separation (16,17). TRLC also proves able to separate closely related diastereomers, as illustrated for a number of peptides in Figure 5(c) (6).
Gradient Analyses
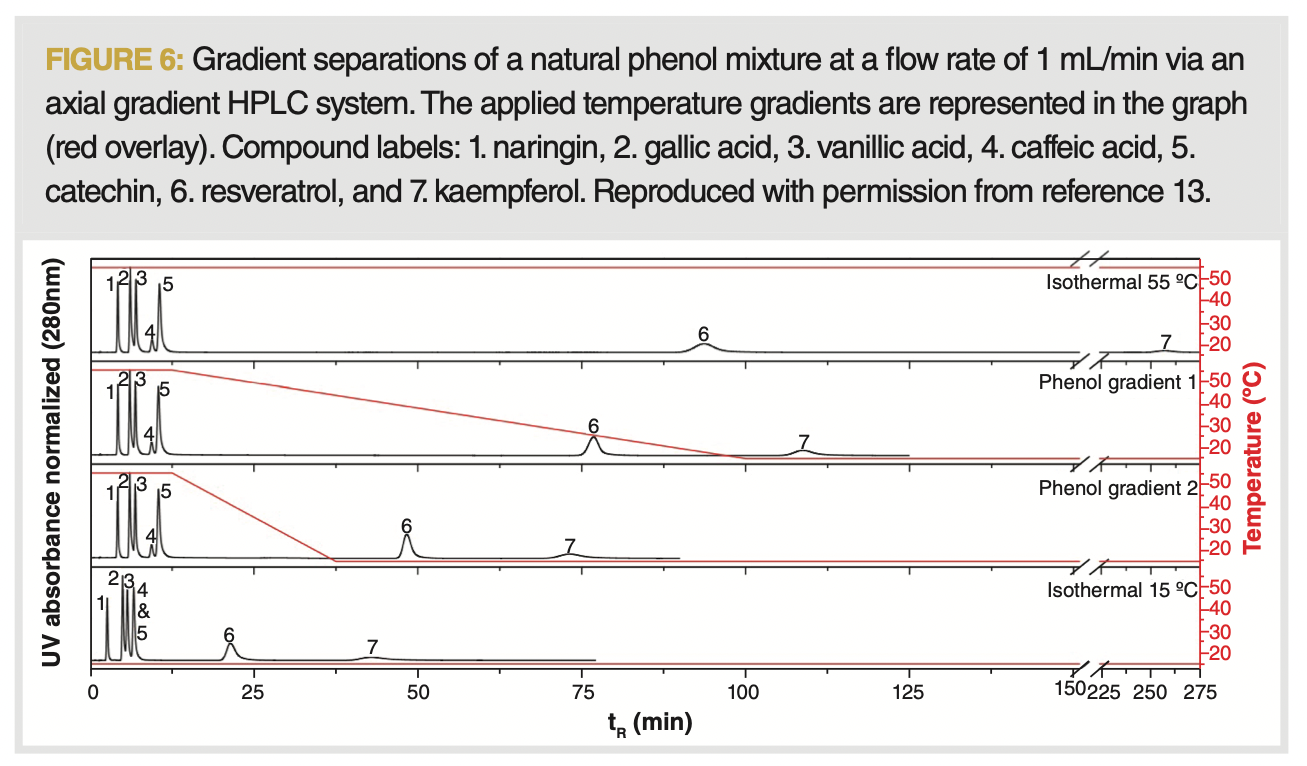
Hydrophobic interaction TRLC is mainly operated at temperatures above the LCST, allowing exploitation of the then-obtained higher retention, while benefiting of reasonable plate numbers and selectivity reachable under those conditions. The main benefits of TRLC can be reaped when this is combined with a downward temperature gradient imposed on the columns. Although the column temperature can also be programmed in conventional static air ovens, their thermal mass hinders imposing rapid cooling gradients. Programmable water baths are mostly used to control the column temperature in TRLC, as they allow accurate temperature control. Recently, we introduced a column cooling approach, based on the controlled mixing of a hot and cold mobile phase as reachable through any binary HPLC pump, allowing for faster gradients as compared to the use of programmable water baths (13). The ensuing drastic reduction in retention time and obtained gradient peak focusing are illustrated in Figure 6.
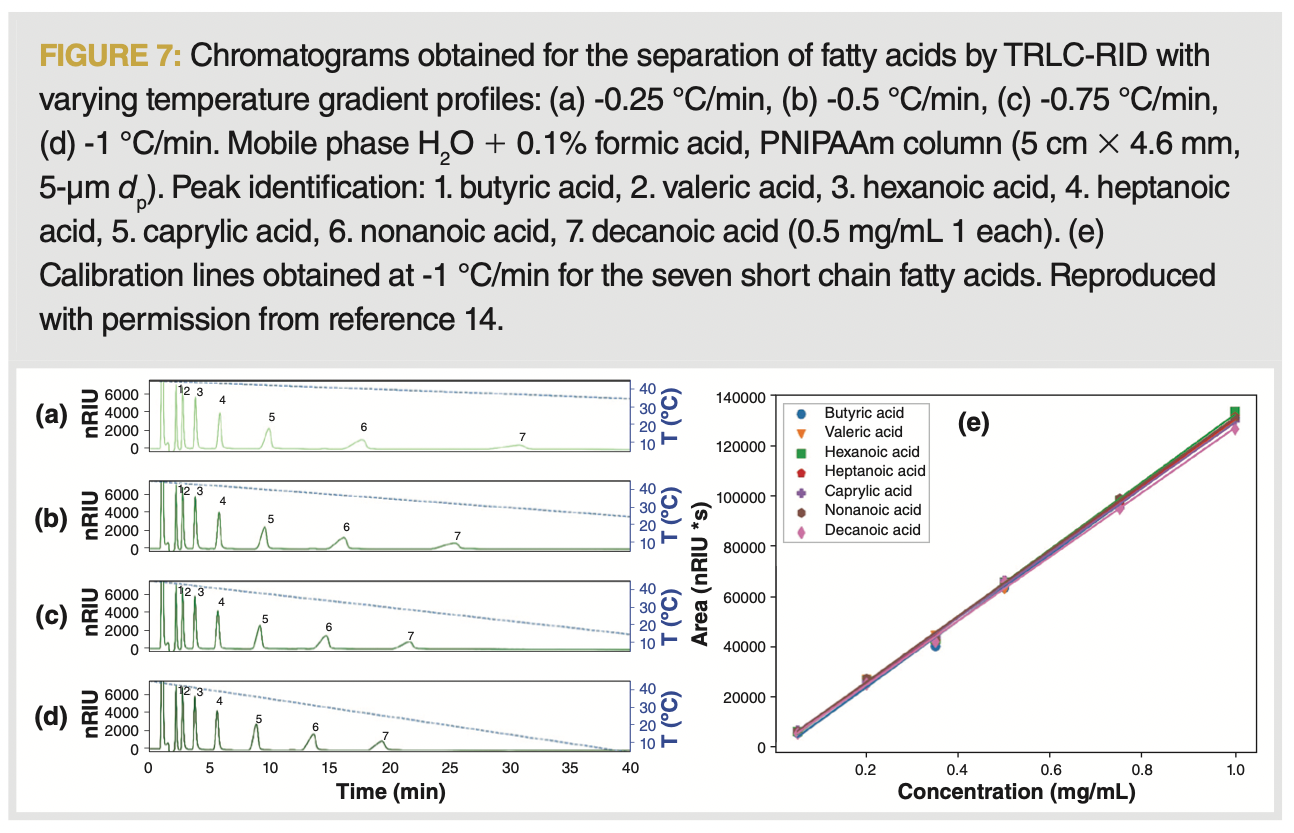
The use of gradients also allows for novel possibilities in the field of quantitative analysis. While the combination of compositional solvent gradients with refractive index (RI) detection is prohibitive, the use of the thermal gradients required in TRLC proves not to influence the noise, drift, or response of this detector. A quantitative application is shown for a series of short chain fatty acids determined under the steepest gradient conditions (45 to 5 °C at –1°/min). The overlapping calibration lines illustrate that standard independent quantitation now becomes possible, while the applicability range of the RI detector is extended (Figure 7).
Summary
TRLC is an alternative separation mode that makes it possible to obtain separations similar to those of reversed‑phase LC while using only water as the mobile phase. Although poly(N-isopropylacrylamide) (PNIPAAm) is still the most commonly used polymer in TRLC, the possibilities offered by other temperature-responsive acrylamides is appealing, and potentially more interesting, because they offer more retention or dehydrate at lower temperatures. The efficiency of the columns obtained via such polymers immobilized on silica particles appears not fundamentally hindered and an evolution towards more efficient columns appears realistic. Nevertheless, the bulky nature of a polymer layer on silica slows down mass transfer and the resulting optimal velocities. The selectivity of TRLC columns differs between the polymers and at different temperatures. TRLC is most useful when it is used under thermal gradient conditions that allow for peak focusing and reduced analysis times. It could also be shown that the use of temperature gradients in TRLC is compatible with RI detection, allowing for novel applications via this fairly universal detector.
References
- Kanazawa, H.; Yamamoto, K.; Kashiwase, Y.; et al. Analysis of Peptides and Proteins by Temperature-Responsive Chromatographic System Using N-Isopropylacrylamide Polymer-Modified Columns. J. Pharm. Biomed. Anal. 1997, 15, 1545–1550. DOI: 10.1016/S0731-7085(96)02004-3
- Kanazawa, H.; Yamamoto, K.; Matsushima, Y.; et al. Temperature-Responsive Chromatography Using Poly(N-Isopropylacrylamide)-Modified Silica. Anal. Chem. 1996, 68, 100–105. DOI: 10.1021/ac950359j
- Ampe, A.; Wicht, K.; Baert, M.; et al. Investigation of the Potential of Mixed Solvent Mobile Phases in Temperature-Responsive Liquid Chromatography (TRLC). Analyst 2021, 146, 6990–6996. DOI: 10.1039/D1AN01684A
- Baert, M.; Wicht, K.; Hou, Z. Y.; et al. Exploration of the Selectivity and Retention Behavior of Alternative Polyacrylamides in Temperature Responsive Liquid Chromatography. Anal. Chem. 2020, 92, 9815–9822. DOI: 10.1021/acs.analchem.0c01321
- Baert, M.; Martens, S.; Desmet, G.; et al. Enhancing the Possibilities of Comprehensive Two-Dimensional Liquid Chromatography through Hyphenation of Purely Aqueous Temperature-Responsive and Reversed-Phase Liquid Chromatography. Anal. Chem. 2018, 90, 4961–4967. DOI: 10.1021/acs.analchem.7b04914
- Satti, A. J.; Espeel, P.; Martens, S.; et al. Tunable Temperature Responsive Liquid Chromatography Through Thiolactone-Based Immobilization of Poly(N-Isopropylacrylamide). J. Chromatogr. A 2015, 1426, 126–132. DOI: 10.1016/j.chroma.2015.11.063
- Miserez, B.; Lynen, F.; Wright, A.; Euerby, M.; Sandra, P. Thermoresponsive Poly(N-vinylcaprolactam) as Stationary Phase for Aqueous and Green Liquid Chromatography. Chromatographia 2010, 71, 1–6. DOI: 10.1365/s10337-009-1394-3
- Lynen, F.; Heijl, J. M. D.; Du Prez, F. E.; et al. Evaluation of the Temperature Responsive Stationary Phase Poly(N-isopropylacrylamide) in Aqueous LC for the Analysis of Small Molecules. Chromatographia 2007,66, 143–150. DOI: 10.1365/s10337-007-0301-z
- Lorenzo, R. A.; Carro, A. M.; Concheiro, A.; Alvarez-Lorenzo, C.; Stimuli-Responsive Materials in Analytical Separation. Anal. Bioanal. Chem. 2015, 407, 4927–4948. DOI: 10.1007/s00216-015-8679-1
- Aseyev, V.; Tenhu, H.; Winnik, M. Non-Ionic Thermoresponsive Polymers in Water. In Self Organized Nanostructures of Amphiphilic Block Copolymers II, Muller, A. H. E., Borisov, O., Eds.; Springer, 2011; pp. 29–89.
- Linford, M.; Jensen, D. S.; Clark, J.; Teutenberg, T. Elevated Temperatures in Liquid Chromatography, Part III: A Closer Look at the van ‘t Hoff Equation. LCGC North America 2012, 30, 1052–1057.
- Petre, J.; Iancu, V.; David, V. Rev. Roum. Biochim. 2013, 58, 425–432.
- Baert, M.; Wicht, K.; Moussa, A.; et al. Implementations of Temperature Gradients in Temperature-Responsive Liquid Chromatography. J. Chromatog. A 2021, 1654, 1–11. DOI: 10.1016/j.chroma.2021.462425
- Bandini, E.; Wicht, K.; Ampe, A.; et al. Hyphenating Temperature Gradient Elution with Refractive Index Detection Through Temperature-Responsive Liquid Chromatography. Anal. Chim. Acta 2022, 1231, 340441. DOI: 10.1016/j.aca.2022.340441
- Vanhoenacker, G.; Pereira, A. D.; Kotsuka, T.; et al. Evaluation of a New Polymeric Stationary Phase with Reversed-Phase Properties for High Temperature Liquid Chromatography. J. Chromatog. A 2010, 1217, 3217–3222. DOI: 10.1016/j.chroma.2009.09.070
- De Beer, M.; Lynen, F.; Chen, K.; et al. Stationary-Phase Optimized Selectivity Liquid Chromatography: Development of a Linear Gradient Prediction Algorithm. Anal. Chem. 2010, 82, 1733–1743. DOI: 10.1021/ac902287v
- Lynen, F.; Hegade, R.; De Beer, M.; et al. Stationary-Phase Optimized Selectivity in Liquid Chromatography (SOS-LC) for Pharmaceutical Analysis. LCGC Europe 2018, 31, 82–89.
ABOUT THE COLUMN EDITOR
David S. Bell is a research fellow in Research and Development at Restek.
ABOUT THE AUTHORS
Frédéric Lynen is a professor at Ghent University, Ghent, Belgium, and director of the Separation Science Group.
Adriaan Ampe is a PhD researcher at Ghent University.
Elena Bandini is a PhD researcher at Ghent University.
Mathijs Baert completed his PhD research at Ghent University.
Kristina Wicht completed her PhD research at Ghent University.
Ardiana Kajtazi is a PhD researcher at Ghent University.
Turaj Rahmani is a PhD researcher at Ghent University.
Jonas Veenhoven is a PhD researcher at Ghent University.
Gaëlle Spileers is a PhD researcher at Ghent University.

Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.












