Enhancing Peptide Mapping Sequence Coverage Through an Automated Dual Protease Digest
Peptide mapping is routinely used in the characterization of monoclonal antibodies (mAbs) for confirmation of the primary sequence and for the detection of post-translational modifications (PTMs). Trypsin is one of the most commonly used proteases in peptide mapping protocols due to its high level of specificity. However, it has been observed that trypsin alone is not always sufficient for full sequence coverage because of the presence of long sequences of hydrophobic amino acids that lack trypsin-specific cleavage sites. In this article, trypsin was combined with chymotrypsin to overcome this loss of sequence coverage. Through the immobilization of these proteases on magnetic beads, and by performing the digestion using an automated platform, a rapid and reproducible digest was achieved with low levels of nonspecific peptides (< 1.3%) and a low number of unique peptides generated across technical replicates (< 6). By using a ratio of 50:50 (v/v) trypsin–chymotrypsin, full sequence coverage was achievable.
Image Credit: © sakurra - stock.adobe.com
KEY POINTS
- Combining chymotrypsin with traditional trypsin digest can improve sequence coverage in the presence of long hydrophobic peptides.
- The typsin–chymotrypsin ratio needs optimization to maintain low levels of nonspecific cleavages.
- Immobilizing proteases on magnetic beads allows for a higher reproducibility.
Monoclonal antibodies (mAbs) are Y-shaped glycoproteins that consist of two identical heavy chains and two identical light chains that amount to a molecular mass of approximately 150,000 Da (1–3). The light and heavy chains are connected through a series of intra- and intermolecular disulfide bonds that stabilize the structure (4). For immunoglobulin G (IgG) mAbs, the heavy chain consists of four domains, one variable domain (VH), and three constant domains (CH1, CH2, CH3), while the light chain consists of just one variable domain (VL) and one constant domain (CL). VL and CL pair with the VH and CH1 to form the fragment antigen binding (Fab) domain. The location of the two variable domains in the Fab is considered the Fv region. In this region, there are six complementarity-determining sites, which are unique depending on the target antigen for binding; as such, the number of amino acids and the sequence of the complementarity-determining region (CDR) varies (5).
Peptide mapping is one of the most commonly applied analytical tools for the characterization of monoclonal antibodies because it provides site-specific information on post-translational modifications (PTMs), such as the relative abundance levels of oxidation, deamidation, N- and C-terminal composition, glycosylation, isomerization, and glycation. It is also applied for the confirmation of the primary amino acid sequence. Confirmation of the primary sequence and the determination of PTMs present are both characterization requirements under the International Council for Harmonization (ICH) guidelines Q6B (6). Traditional peptide mapping protocols involve reduction and alkylation of disulfide bonds followed by digestion of the mAb using a protease—typically trypsin—before peptide separation using reversed-phase liquid chromatography (RPLC) coupled with mass spectrometry (MS) (7,8). The acquired data are then analyzed offline using software algorithms. A drawback with the traditional methods used for peptide mapping is that they are typically time-consuming and can result in sample preparation-induced modifications. A number of studies have been performed to optimize the time required and reduce the input of process-induced modifications by making the sample preparation process more automated (9–12). Traditional tryptic digests can be insufficient for the characterization of some proteins because of an abundance or lack of tryptic cleavage sites within the primary sequence. Trypsin is a highly specific protease that cleaves proteins at the C-terminal of lysine and arginine (13). However, if these residues are not present in regions characterized by higher hydrophobicity, the resulting peptides might be difficult to track using reversed-phase chromatography for LC–MS analysis because of either the size or resulting hydrophobicity of the peptides formed following protease digestion. As a consequence, the sequence coverage obtained through peptide mapping might be incomplete, preventing the monitoring of critical quality attributes (CQAs) (14). To mitigate this effect, different strategies can be undertaken, including optimizing mobile phase strength and temperature or choosing alternative stationary phase chemistries, such as C4 rather than the widely used C18. Other strategies could involve alternative sample preparation procedures, for example, with the use of other proteases or a combination of more proteases, which could help to obtain smaller peptides that could be more easily monitored. Numerous studies have been published that utilize a combination of proteases in a single digest reaction to improve peptide mapping efficiency. One study compared the result of a double trypsin digest to a surfactant‑assisted digest and a combined LysC and trypsin digest. It was found that combining LysC and trypsin improved the digestion efficiency and reduced missed cleavages, despite the proteases sharing a common specificity in cleavage site (15). The combination of trypsin and chymotrypsin has been successful in the detection of sequence variants in recombinant human mAbs and in the identification of the location of epitopes for a number of mAbs that were shown to react with proteins detected in the brains of Alzheimer’s patients (16,17).
In this study, trypsin and chymotrypsin were combined in a single digestion step using an automated workflow. Chymotrypsin is less specific compared to trypsin. Chymotrypsin preferentially cleaves the C-terminal of aromatic amino acids tryptophan, tyrosine, and phenylalanine; however, it has additional activity on leucine, methionine, and histidine amino acids (18). Due to its lower level of specificity, peptide mapping protocols using chymotrypsin may suffer from a lack of reproducibility and the digestion pattern can be altered when the digestion time is increased. Here, the proteases were immobilized on magnetic beads to allow for a time controlled, robust, and reproducible digest. The primary benefit is that during the immobilization process the proteases are chemically modified in such a way that they are stabilized while maintaining their specificity, and the selectivity of the cleavage site is not affected. On the other hand, the immobilization of the protease prevents them from autolysis, contrary to what is often observed using in‑solution digestion (19,20,21). Using an optimized digestion protocol for the automated trypsin digestion (11), the implementation of chymotrypsin was evaluated using a human recombinant IgG1 mAb with known hydrophobic stretches within the amino acid sequence of its CDR. The sequence coverage and relative PTMs abundance were investigated by comparing a tryptic digestion to a dual protease digest containing various ratios of trypsin and chymotrypsin. Different ratios of trypsin to chymotrypsin were investigated to determine at what ratio full sequence coverage could be obtained while minimizing the levels of nonspecific modifications generated from chymotrypsin. The optimized digest was then assessed to prove robustness and reproducibility of the sample preparation.
Experimental
Materials:
The human IgG1 monoclonal antibody investigated in this study was kindly provided by Symphogen. Thermo Scientific Smart Digest kits (magnetic resin option) for both trypsin and chymotrypsin and Thermo Scientific Bond Breaker Tris(2-carboxyethyl) phosphine hydrochloride (TCEP) solution neutral pH were acquired from Thermo Fisher Scientific. LC–MS-grade 0.1% (v/v) formic acid in water was used as mobile phase A, while LC–MS-grade 0.1% (v/v) formic acid in acetonitrile was used as mobile phase B. Both mobile phase solutions were sourced from Thermo Fisher Scientific. Trifluoroacetic acid was acquired from Sigma Aldrich.
Peptide Mapping Protocol:
Peptide mapping was performed using a Kingfisher Duo Prime Purification System under the control of BindIt software (Thermo Fisher Scientific).
A 100 μg measure of mAb was diluted to 1 mg/mL using high purity water from a Sartorius Arium Purification system, Smart Digest Buffer, and 5 mM TCEP (final concentration). A total volume of 15 μL of magnetic protease beads was used at different ratios of trypsin to chymotrypsin. The following ratios were investigated: 100:0, 75:25, 50:50, and 25:75 (v/v) trypsin–chymotrypsin, respectively. Protease beads were diluted in 100 μL of digest buffer and washed in a 1:4 (v/v) ratio of trypsin digestion buffer in water before being added to the mAb sample. Digestion was performed at 70 °C for 30 min at medium mixing speed. At the end of the digest, the beads were removed from the samples and the reaction was acidified to a final concentration of 0.1% trifluoroacetic acid (TFA) (Sigma Aldrich) before LC–MS analysis.
LC–MS Instrumentation and Parameters:
LC–MS analysis was performed on a Thermo Scientific Vanquish Flex Binary UHPLC system coupled online to a Thermo Scientific Q Exactive Plus Hybrid Quadrupole‑Orbitrap mass spectrometer using a heated electrospray ionization (HESI) source (Thermo Fisher Scientific). Peptide separation was performed using a 2.1 × 250 mm, 2.2-μm Thermo Scientific Vanquish Acclaim C18 column. The peptide mapping gradient was as follows: 2% B to 45% B in 45 min, increased to 90% B at 46 min, and held at 90% B for four min. This was then increased to 2% B at 51 min until 53 min, and increased to 90% B at 54 min until 56.5 min. Requilibration was then followed at 2% B from 56.5 min until the end of the run at 60 min. The mobile phase flow rate was maintained at 0.300 mL/min for the duration of the run and the column temperature was 25 °C. The first two min and the last 12 min of the gradient were diverted to waste using an external valve.
MS tune parameters were set as follows: sheath gas flow rate was 40 AU, auxiliary gas flow rate was 10 AU, spray voltage was 3.80 kV, capillary temperature was 320 °C, S-lens RF voltage level was set to 50.0, and the auxiliary gas heater temperature was 400 °C.
Full MS was acquired at 70,000 resolution (at 200 m/z), with an acquisition gain control (AGC) target of 3 × 106 ions and a maximum injection time of 100 ms. One microscan was performed and a scan range of 200–2000 m/z was used. Data‑dependent MS/MS was performed at 17,500 resolution (at 200 m/z), with an AGC target of 1 × 105 ions and a maximum injection time of 200 ms. One microscan was performed and a loop count of five, MSX count of one, and a topN of five were also applied. Normalized collision energy of 28 and an isolation window of 2.0 m/z were also used.
Data Acquisition and Analysis:
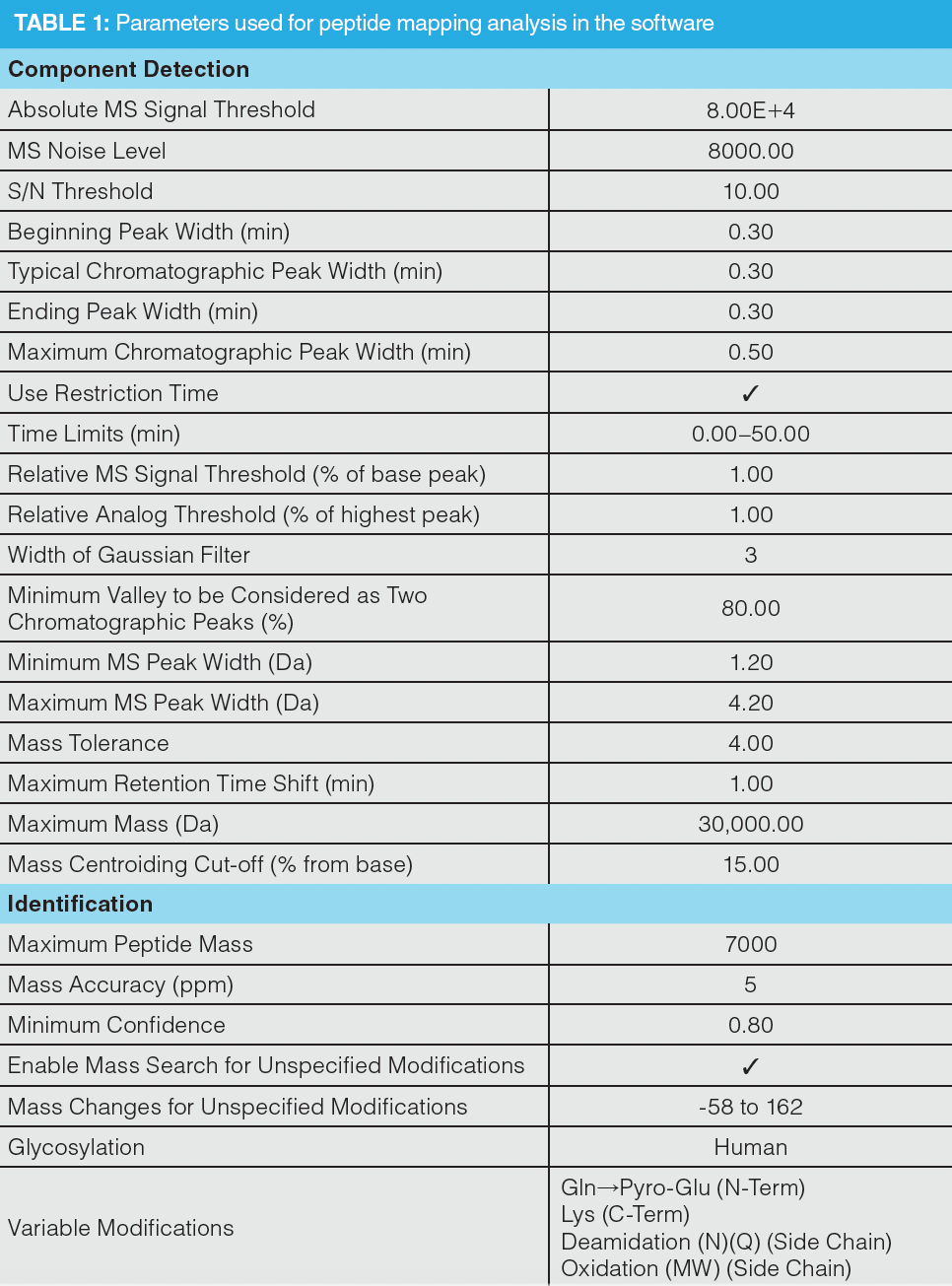
Data were acquired using Thermo Scientific Chromeleon Chromatography Data System (CDS) software 7.2.9 (Thermo Fisher Scientific). Peptide sequence coverage analysis and PTM detection was performed using Thermo Scientific BioPharma Finder software version 4.1. The parameters used are highlighted in Table 1. Peptides were filtered to include a confidence score of ≥ 95%, to be within a delta of ± 5 ppm and to exclude peptides containing Na+/K+ adducts, gas phase ions, nonspecific, and unknown modifications. For trypsin, an additional filter of ≤ 1 missed cleavage was applied because of the high specificity of trypsin. For the combined proteases this was not a viable filter, as there were additional cleavage sites to consider, even within traditional tryptic peptides. For example, DTLMISR, a commonly investigated peptide for the calculation of oxidation abundance, would have two potential missed cleavages when using chymotrypsin. To overcome this, a best overall average structural resolution (ASR) filter of ≥ 1.0 ≤ 1.6 was applied, which resulted in peptides with good quality MS/MS spectra containing sufficient b- and y-diagnostic ions for confirmation of identity. This value can be observed in the fragment coverage map for any detected peptide that contains MS2 scans. However, to assess N-glycosylation, the used filters were based on the peptides EEQYNSTYR, TKPREEQYNSTYR, and EEQYNSTYRVVSVLTVLHQDWLNGK, as they contain N-glycan sites and the signals showed good intensity values. A confidence score of ≥ 95% and accuracy within ± 5 ppm were again selected.
Results and Discussion
Sequence Coverage:
A high level of sequence coverage in peptide mapping protocols is vital to ensure accurate detection of site-specific modifications and for confirmation of sequence in critical regions such as the CDR (10). The results from this investigation demonstrate that for the IgG1 used, the use of trypsin alone was not sufficient to obtain full sequence coverage (Figure 1). To overcome this loss of sequence coverage, an increasing amount of chymotrypsin was combined with trypsin to yield ratios of 75:25, 50:50, and 25:75 (v/v) trypsin–chymotrypsin, respectively. The results from this investigation show that when using ratios containing ≥ 50% chymotrypsin, full sequence coverage was obtained (Figure 1). Further investigation into the generation of nonspecific peptides with different ratios of trypsin to chymotrypsin showed that when the percentage of chymotrypsin was ≥ 50%, the relative abundance and number of nonspecific‑generated peptides did not exceed those generated in trypsin alone (Figure 2). The levels of nonspecific cleavage were lower than those previously reported in our interlaboratory study using immobilized trypsin alone (11).
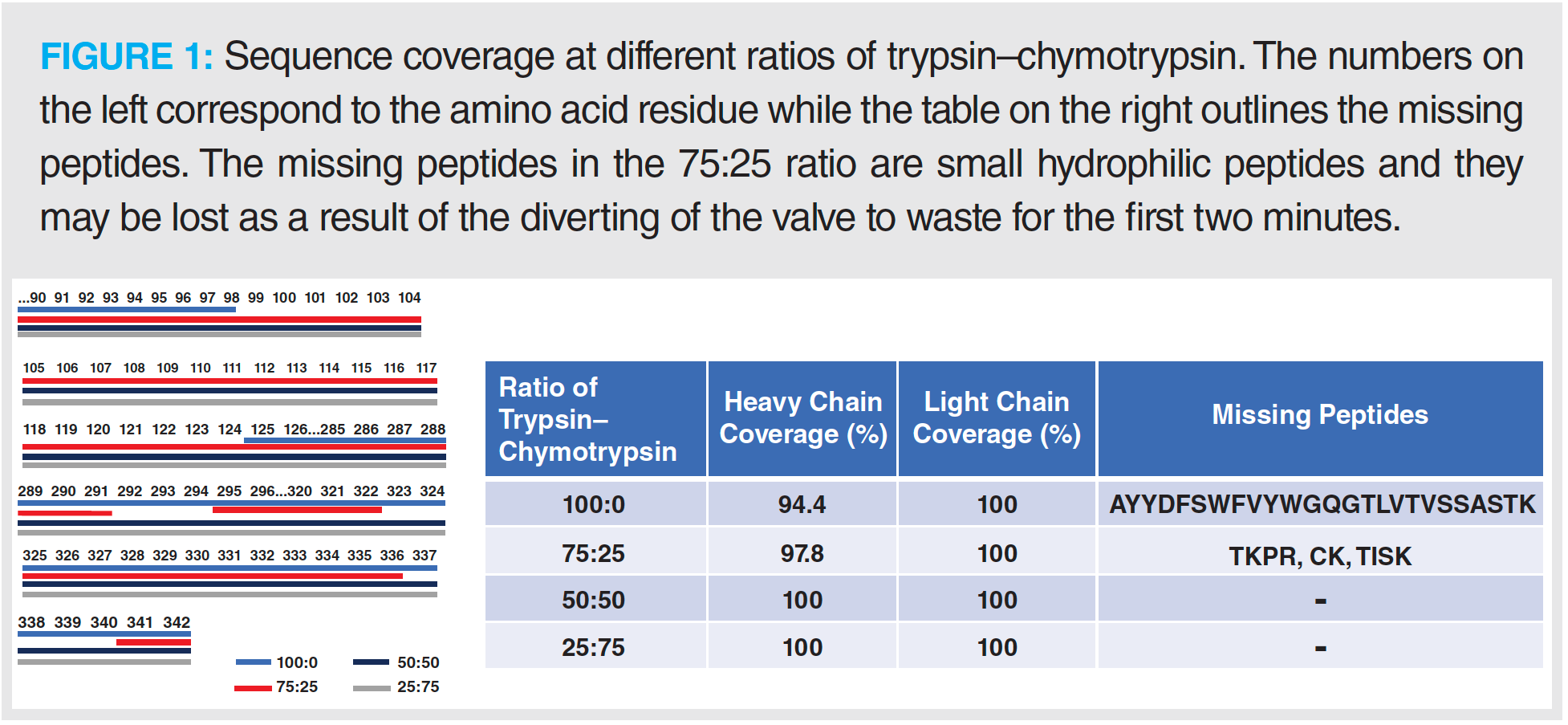
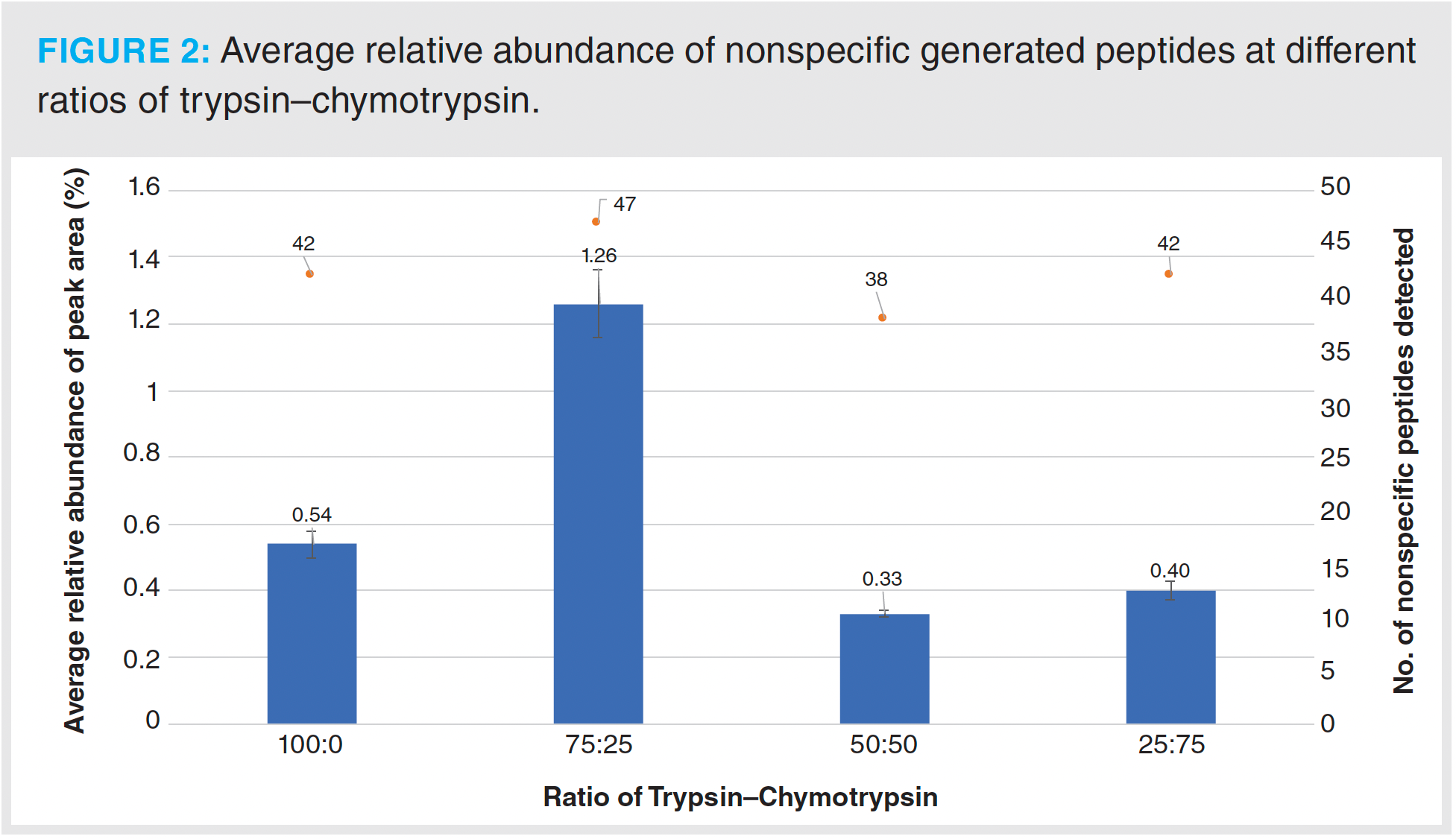
As smaller peptides can be more hydrophilic and thus more difficult to retain on reversed-phased chromatographic supports, excessive digestion of the sample should also be avoided to achieve full sequence coverage. In this study, when adding chymotrypsin, a sharp increase of peptides consisting of three or four amino acids was observed while full sequence coverage was achieved; this was the result of overlapping peptides and good retention of the short peptides. Digestion conditions may need optimization for different analytes or when using a different separation setup.
PTM Abundance:
As previously mentioned, peptide mapping is a powerful analytical technique used for the detection of site-specific modifications. Using the different ratios of proteases, the average relative abundance (n = 3) of a number of CQAs was investigated using a tryptic digest as a reference for comparative purposes. Figure 3 shows the average relative abundance for methionine oxidation (Figure 3[a]), asparagine and glutamine deamidation (Figure 3[b]), and N-terminal pyroglutamate formation and C-terminal lysine content (Figure 3[c]).

Oxidation is an important CQA to monitor because it can induce structural changes within the mAb and can result in decreased efficacy, safety, and stability (22). Oxidation commonly occurs in methionine residues, in particular at sites approximately located at M252 and M428 in the constant heavy chain region; however, oxidation has also been observed in tryptophan residues in the CDR, which can result in reduced or even abolished antigen binding (23,24). The average relative abundance of oxidation was lower in samples digested with a ratio of chymotrypsin ≥ 50:50 compared to the control for M48 and M254, while only a slight increase was recorded for M430. For all samples—including the control—the average relative abundance of each site was below 4%. The relative standard deviation (RSD) for all sites was below 10%, except for M48 using 50:50 where an RSD of 11.29% was determined.
Deamidation of mAbs can result in protein degradation and limit shelf life. Deamidation rates are susceptible to factors such as buffer concentration, pH, and storage temperatures (25). Deamidation commonly occurs at asparagine residues; however, the location of specific deamidation events can affect biological function and activity. For example, a recently published study found that deamidation at N325 abolished antibody-dependent cellular cytotoxicity (ADCC) activity through the disruption of the binding interface between the Fc and the FcγRIIIa receptor (26). Deamidation in the CDRs of mAbs has also been shown to result in a loss of activity (13). In this study, detection of deamidated peptides was enhanced through the use of a 250 mm column, as deamidated peptides often coelute with native peptides (27). The average relative abundance of deamidation detected was lower using ratios of chymotrypsin ≥ 50:50 compared to the trypsin-only reference, with the exception of N327. Overall, the average relative abundance of all detected deamidation events was less than 0.6%.
Different ratios of trypsin–chymotrypsin had little to no impact on the levels of average relative abundance recorded for pyroglutamate formation and lysine content as expected. The RSD for both PTMs did not exceed 1.5%, demonstrating good levels of reproducibility across all replicates and ratios.
The N-glycan profile of the IgG1 mAb was also monitored using different ratios of the two proteases. N-glycosylation is an important PTM, as it lends stability to the IgG molecule, and alterations in glycosylation can result in a loss of biological activity based on conformational changes in the Fc region (28,29). Figure 4 shows the average relative abundance for the glycans detected. The main glycoform detected across all ratios was A2G0F, the core fucosylated agalactosyl biantennary glycan. Overall, the determination of the relative abundance of the 12 N-glycans detected was not affected by the different conditions used for protein digestion (Figure 4).
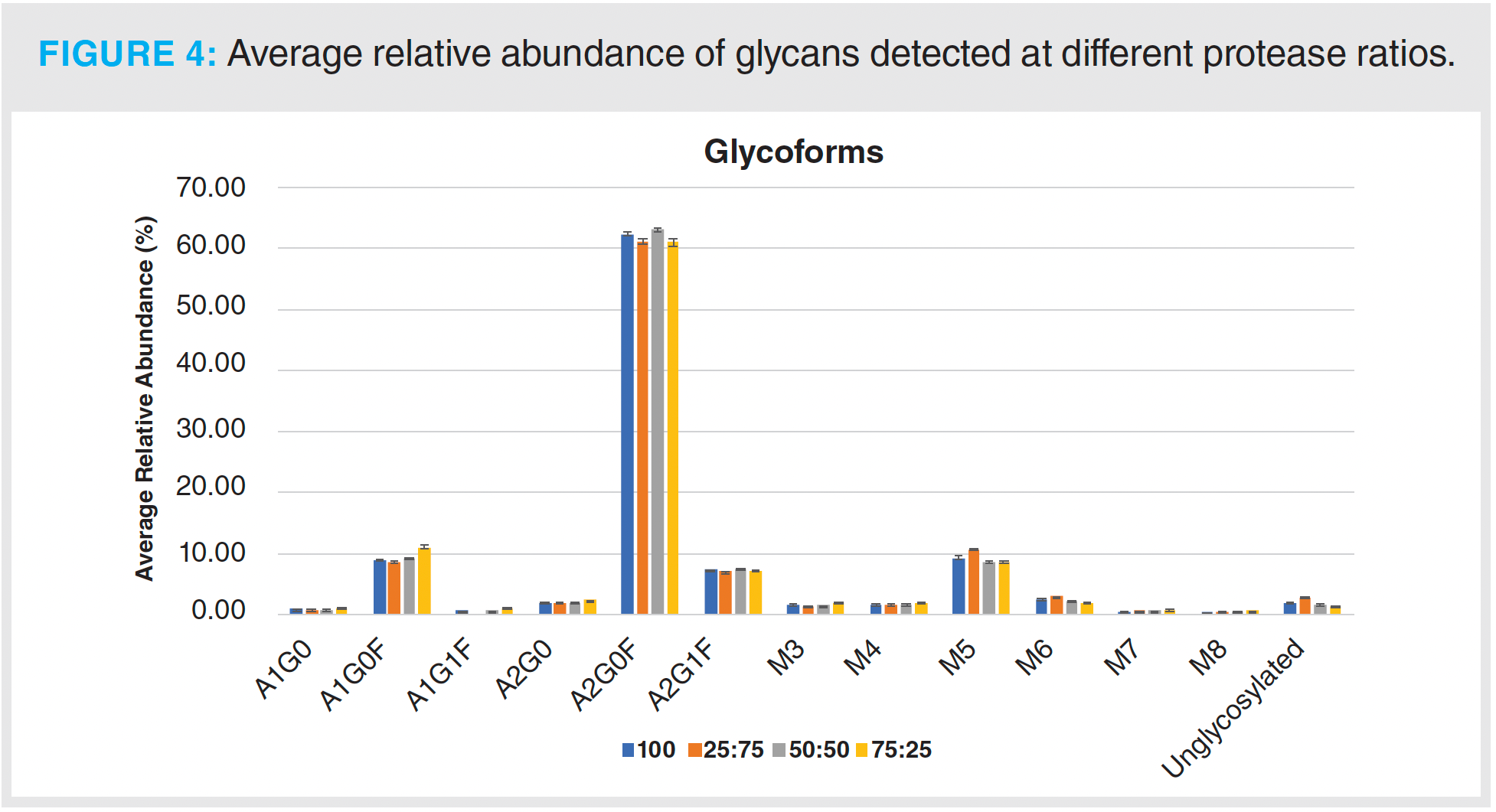
The results from this initial analysis show that using a protease ratio of 50:50 was sufficient to obtain full sequence coverage and did not have an overall negative impact on the abundance levels of the monitored PTMs.
Method Reproducibility:
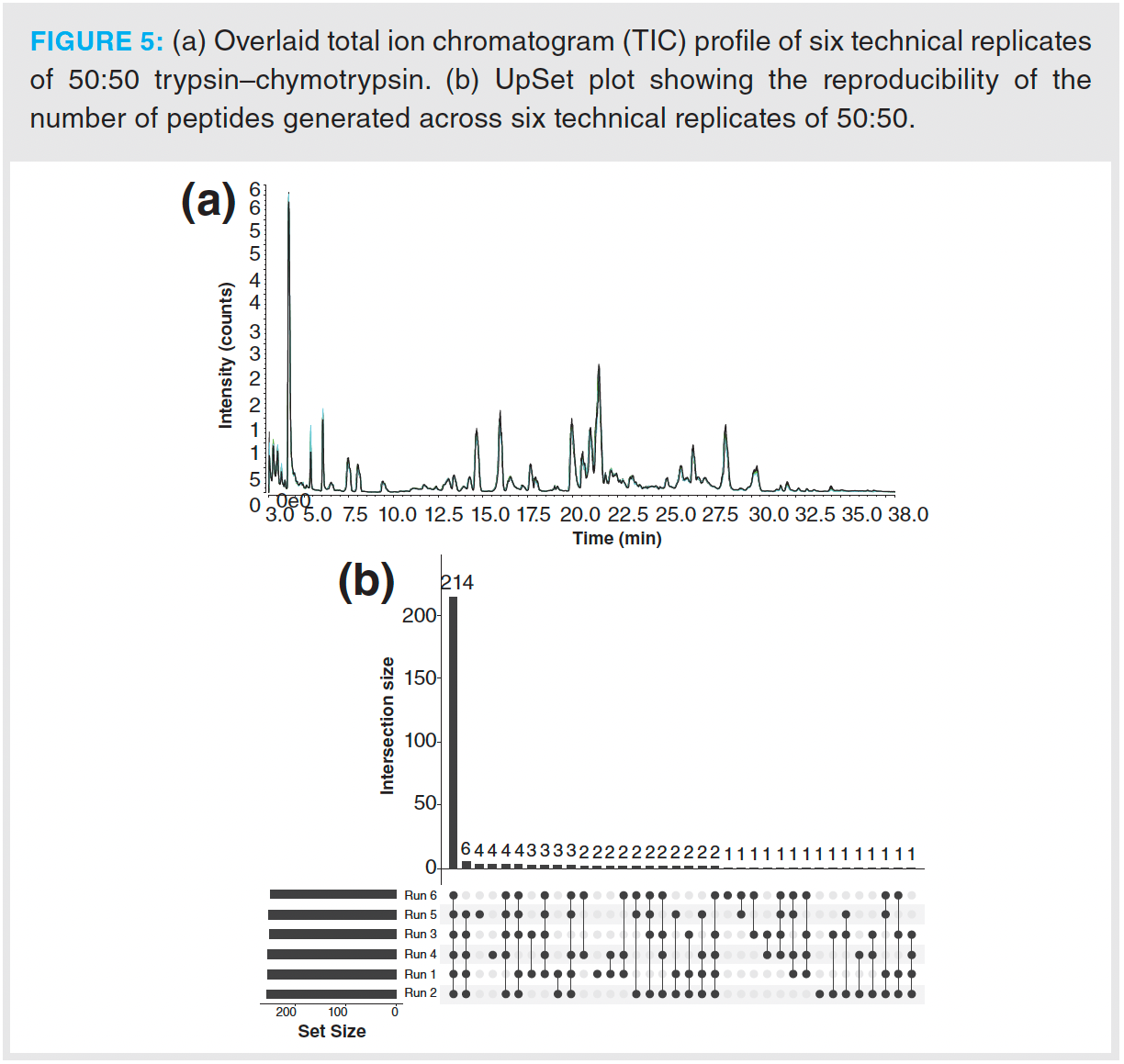
To ensure method reproducibility, six technical replicates were performed using a protease ratio of 50:50. The overlaid total ion chromatogram (TIC) of these six replicates is shown in Figure 5(a). The number of unique and common peptides generated across this sample set was visualized using an UpSet plot (Figure 5[b]) (30). This relationship plot shows that all six replicates had over 210 peptides in common and that the number of unique peptides generated was low; no more than four unique peptides were detected in a single run, demonstrating a high level of reproducibility across the digestion procedures based on automated workflows.
Conclusions
Peptide mapping remains the gold standard for understanding the abundance of site-specific modifications and for the confirmation of the primary protein sequence. This study has examined the applicability of using dual proteases with an automated digestion technique. First, different ratios of trypsin to chymotrypsin were investigated and full sequence coverage was achieved when using protease ratios containing ≥ 50% chymotrypsin. The findings from this study correspond well with previously published studies; the first found that a combination digest of trypsin and chymotrypsin improved sequence coverage from ~88% when using trypsin alone to 98.5% (17). The ratio of trypsin to chymotrypsin was supported by the second study, which used a similar ratio of proteases and was able to improve the detection of hydrophobic proteins (14). The average relative abundance of PTMs, such as oxidation, deamidation, and N-glycosylation, was measured in all protease ratios and compared to the levels observed when using trypsin alone as a reference. It was found that when using a protease ratio of 50:50, the levels of PTMs detected were comparable to those in the control. Method reproducibility was investigated next using six technical replicates. The method was shown to have a high level of reproducibility with regards to retention time. The very low level of nonspecific peptides generated highlights its suitability for workflows such as the multi-attribute method (MAM) for molecules, where digestion with trypsin alone is not sufficient. Automation of the digestion further paves the way for dual protease digestion to be applied in quality control (QC) environments.
References
(1) Beck, A.; Liu, H. Macro- and Micro- Heterogeneity of Natural and Recombinant IgG Antibodies. Antibodies (Basel) 2019, 8 (1), 18. DOI: 10.3390/antib8010018
(2) Xu, Y.; Wang, D.; Mason, B.; et al. Structure, Heterogeneity and Developability Assessment of Therapeutic Antibodies. MAbs 2019, 11 (2), 239–264. DOI: 10.1080/19420862.2018.1553476
(3) Graf, T.; Heinrich, K.; Grunert, I.; et al. Recent Advances in LC-MS Based Characterization of Protein-Based Bio-Therapeutics - Mastering Analytical Challenges Posed by the Increasing Format Complexity. J. Pharm. Biomed. Anal. 2020, 186, 113251. DOI: 10.1016/j.jpba.2020.113251
(4) Vidarsson, G.; Dekkers, G.; Rispens, T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014, 5, 520. DOI: 10.3389/fimmu.2014.00520
(5) Vlasak, J.; Bussat, M.C.; Wang, S.; et al. Identification and Characterization of Asparagine Deamidation in the Light Chain CDR1 of a Humanized IgG1 Antibody. Anal. Biochem. 2009, 392 (2), 145–54. DOI: 10.1016/j.ab.2009.05.043
(6) ICH, Q6B Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products, (1999).
(7) Qu, M.; An, B.; Shen, S.; et al. Qualitative and Quantitative Characterization of Protein Biotherapeutics with Liquid Chromatography Mass Spectrometry. Mass Spectrom. Rev. 2017, 36 (6), 734–754. DOI: 10.1002/mas.21500
(8) Rathore, D.; Faustino, A.; Schiel, J.; et al. The Role of Mass Spectrometry in the Characterization of Biologic Protein Products. Expert Rev. Proteomics 2018, 15 (5), 431–449. DOI: 10.1080/14789450.2018.1469982
(9) Richardson, J.; Shah, B.; Xiao, G.; Bondarenko, P.V.; Zhang, Z. Automated In-Solution Protein Digestion Using a Commonly Available High-Performance Liquid Chromatography Autosampler. Anal. Biochem. 2011, 411 (2), 284–91. DOI: 10.1016/j.ab.2011.01.019
(10) Mouchahoir, T.; Schiel, J.E. Development of an LC-MS/MS Peptide Mapping Protocol for the NISTmAb. Anal. Bioanal. Chem. 2018, 410 (8), 2111–2126. DOI: 10.1007/s00216-018-0848-6
(11) Millan-Martin, S.; Jakes, C.; Carillo, S.; et al. Inter-Laboratory Study of an Optimised Peptide Mapping Workflow Using Automated Trypsin Digestion for Monitoring Monoclonal Antibody Product Quality Attributes. Anal. Bioanal. Chem. 2020, 412 (25), 6833–6848. DOI: 10.1007/s00216-020-02809-z
(12) Qian, C.; Niu, B.; Jimenez, R.B.; Wang, J.; Albarghouthi, M. Fully Automated Peptide Mapping Multi-Attribute Method by Liquid Chromatography-Mass Spectrometry with Robotic Liquid Handling System. J. Pharm. Biomed. Anal. 2021, 198, 113988. DOI: 10.1016/j.jpba.2021.113988
(13) Schmid, I.; Bonnington, L.; Gerl, M.; et al. Assessment of Susceptible Chemical Modification Sites of Trastuzumab and Endogenous Human Immunoglobulins at Physiological Conditions. Commun. Biol. 2018, 1, 28. DOI: 10.1038/s42003-018-0032-8
(14) Vermachova, M.; Purkrtova, Z.; Santrucek, J.; et al. Combining Chymotrypsin/Trypsin Digestion to Identify Hydrophobic Proteins from Oil Bodies. Methods Mol. Biol. 2014, 1072, 185–198. DOI: 10.1007/978-1-62703-631-3_14
(15) Glatter, T.; Ludwig, C.; Ahrné, E.; et al. Large-Scale Quantitative Assessment of Different In-Solution Protein Digestion Protocols Reveals Superior Cleavage Efficiency of Tandem Lys-C/Trypsin Proteolysis Over Trypsin Digestion. J. Proteome Res. 2012, 11 (11), 5145–56. DOI: 10.1021/pr300273g
(16) Ksiezak-Reding, H.; Chien, C.H.; Lee, V.M.; Yen, S.H. Mapping of the Alz 50 Epitope in Microtubule-Associated Proteins Tau. J. Neurosci. Res. 1990, 25 (3), 412–9. DOI: 10.1002/jnr.490250319
(17) Yang, Y.; Strahan, A.; Li, C.; et al. Detecting Low Level Sequence Variants in Recombinant Monoclonal Antibodies. MAbs 2010, 2 (3), 285–98. DOI: 10.4161/mabs.2.3.11718
(18) Williams, K.R.; Stone, K.L. Enzymatic Cleavage and HPLC Peptide Mapping of Proteins. Mol. Biotechnol. 1997, 8 (2), 155–67. DOI: 10.1007/BF02752260
(19) Morellon-Sterling, R.; Tavano, O.; Bolivar, J.M.; et al. A Review on the Immobilization of Pepsin: A Lys-Poor Enzyme that is Unstable at Alkaline pH Values. Int. J. Biol. Macromol. 2022, 210, 628–702. DOI: 10.1016/j.ijbiomac.2022.04.224
(20) Sharma, S.; Gupta, S.; Princy; Kumar Arya, S.; Kaur, A. Enzymes Immobilization: Implementation of Nanoparticles and an Insight into Polystyrene as the Contemporary Immobilization Matrix. Process Biochem. 2022, 120, 22–34. DOI: 10.1016/j.procbio.2022.05.022
(21) Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Castañeda-Valbuena, D.; et al. Immobilization of Papain: A Review. Int. J. Biol. Macromol. 2021, 188, 94–113. DOI: 10.1016/j.ijbiomac.2021.08.016
(22) Pavon, J.A.; Li, X.; Chico, S.; et al. Analysis of Monoclonal Antibody Oxidation by Simple Mixed Mode Chromatography. J. Chromatogr. A 2016, 1431, 154–165. DOI: 10.1016/j.chroma.2015.12.068
(23) Stracke, J.; Emrich, T.; Rueger, P.; et al. A Novel Approach to Investigate the Effect of Methionine Oxidation on Pharmacokinetic Properties of Therapeutic Antibodies. MAbs 2014, 6 (5), 1229–42. DOI: 10.4161/mabs.29601
(24) Dashivets, T.; Stracke, J.; Dengl, S.; et al. Oxidation in the Complementarity-Determining Regions Differentially Influences the Properties of Therapeutic Antibodies. MAbs 2016, 8 (8), 1525–1535. DOI: 10.1080/19420862.2016.1231277
(25) Pace, A.L.; Wong, R.L.; Zhang, Y.T.; Kao, Y.-H.; Wang, Y.J. Asparagine Deamidation Dependence on Buffer Type, pH, and Temperature. J. Pharm. Sci. 2013, 102 (6), 1712–1723. DOI: 10.1002/jps.23529
(26) Lu, X.; Machiesky, L.A.; De Mel, N.; et al. Characterization of IgG1 Fc Deamidation at Asparagine 325 and Its Impact on Antibody-Dependent Cell-Mediated Cytotoxicity and FcγRIIIa Binding. Sci. Rep. 2020, 10 (1), 383. DOI: 10.1038/s41598-019-57184-2
(27) Badgett, M.J.; Boyes, B.; Orlando, R. The Separation and Quantitation of Peptides with and without Oxidation of Methionine and Deamidation of Asparagine Using Hydrophilic Interaction Liquid Chromatography with Mass Spectrometry (HILIC-MS). J. Am. Soc. Mass Spectrom. 2017, 28 (5), 818–826. DOI: 10.1007/s13361-016-1565-z
(28) Boune, S.; Hu, P.; Epstein, A.L.; Khawli, L.A. Principles of N-Linked Glycosylation Variations of IgG-Based Therapeutics: Pharmacokinetic and Functional Considerations. Antibodies (Basel) 2020, 9 (2), 22. DOI: 10.3390/antib9020022
(29) Carillo, S.; Pérez-Robles, R.; Jakes, C.; et al. Comparing Different Domains of Analysis for the Characterization of N-Glycans on Monoclonal Antibodies. J. Pharm. Anal. 2020, 10 (1), 23–34. DOI: 10.1016/j.jpha.2019.11.008
(30) Lex, A.; Gehlenborg, N.; Strobelt, H.; Vuillemot, R.; Pfister, H. UpSet: Visualization of Intersecting Sets. IEEE Trans. Vis. Comput. Graph. 2014, 20 (12), 1983–92. DOI: 10.1109/TVCG.2014.2346248
Craig Jakes is a research scientist at NIBRT supporting the development of the MAM workflow.
Silvia Millán-Martín is a senior research scientist at the Characterisation and Comparability Laboratory.
Ken Cook is EU Bio-Separations manager at Thermo Fisher Scientific.
Dan Bach Kristensen is a principal scientist at Symphogen, a part of Servier.
Jonathan Bones is principal investigator of the NIBRT Characterization and Comparability Laboratory and an associate professor in the School of Chemical and Bioprocess Engineering at University College Dublin.
Sara Carillo is the bioanalytical research lead in the Characterisation and Comparability Laboratory in NIBRT.

Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)