Paper-Based Sorptive Phases Prepared by Dip Coating
LCGC Europe
Paper-based sorptive phases are promising tools in sample preparation because of their high surface-to-volume ratio, porosity, and versatility. This article discusses the synthesis of paper-based sorptive phases by dip coating. This procedure has allowed a wide variety of phases coated with polymers, nanoparticles, and their combination to be synthesized. This article presents the synthesis, types of coatings, and extraction devices, to highlight the versatility and potential of these materials to the analytical community, particularly for sample preparation.
Paper-based sorptive phases are promising tools in sample preparation because of their high surface-to-volume ratio, porosity, and versatility. This article discusses the synthesis of paper-based sorptive phases by dip coating. This procedure has allowed a wide variety of phases coated with polymers, nanoparticles, and their combination to be synthesized. This article presents the synthesis, types of coatings, and extraction devices, to highlight the versatility and potential of these materials to the analytical community, particularly for sample preparation.

The development of new sorptive phases is a hot topic in solid-phase microextraction (SPME)-related techniques because it broadens the application scope and opens the door to the resolution of new analytical problems (1,2). Research is focused not only on the chemistry of these phases but also on their geometry (3,4). In this scenario, flat supports emerge as an alternative because they present higher capacity compared, for example, with the classical SPME fibre, and enhanced kinetics as a result of their excellent area-to-volume ratio (5,6). Commercial membranes consisting of a mat of cross-linked polymeric fibres have been traditionally used in solid-phase extraction (SPE), especially when high sample volumes (a typical situation in environmental water analysis) are processed. These membranes can also be loaded with particles, in the so-called extraction disks, to increase their sorption capacity. Although commercial membranes have a high potential, the ad hoc fabrication of membranes is an extended strategy in research laboratories. These laboratory-made membranes can be designed after the evaluation of the analytical problem (binomial analyte–sample) by selecting the best polymer that provides a strong/selective interaction with the analyte in the sample environment. Laboratory-made membranes, which are usually fabricated by electrospinning (7) (and also by casting or spin coating), can consist of a combination of polymers, and they can even be modified with nanoparticles, which are embedded in the polymeric network during the synthesis or loaded after it.
In recent years, new promising flat materials have been proposed. Kabir and Furton from the Florida International University have introduced the so-called fabric phases (FPs) (8). As the name indicates, these materials consist of natural or synthetic fabrics superficially modified by sol-gel reaction to immobilize active sorptive phases. The potential of FPs, which has been extensively demonstrated (9), hinges on the wide variety of existing coatings and their extraction capacity. The flexibility and permeability of FPs is a remarkable bonus that makes their application in air sampling possible (10). Fabric phases are produced by a green method, more environmentally friendly than classical synthesis, showing a relevant trend in the design of extraction phases.
Paper is an excellent support for the fabrication of sorptive phases since it is cheap, easily modified, and porous. Although unmodified paper has been proposed for the extraction of 8-hydroxy-2’-deoxyguanosine from urine samples (11), the modification of its surface with sorptive groups is the strategy of choice to increase its capacity and applicability (12). The chemical modification of paper usually involves covalent bonding, which requires complex synthesis procedures. Up to now, several phases have been proposed as coatings, but polymers are the predominant ones due to their exceptional properties. Ackerman and Hurtubise evaluated the modification of paper with poly(hydrogenmethylsiloxane) and dichlorodimethylsilane for the extraction of aromatic compounds (13). Recently, polydimethylsiloxane has also been evaluated for the preconcentration of volatile organic compounds (VOCs) by headspace analysis (14).
In this article, a general overview of the versatility and potential of paper-based sorptive phases prepared by dip coating is presented. This article will present ideas rather than applications to draw the attention of the analytical chemistry community to the potential of these materials. The specific applications, which can be found in the respective publications (if anyone wants to go more in-depth) covers not only analytical methods but also environmental remediation applications (photocatalysis of dyes). Here, the general synthesis procedure and the type of coatings (involving polymers, nanoparticles, and their combination) that have been proposed up to now are described. The extraction devices used for the practical application of these phases are also presented and discussed.
Polymer-Coated Paper
In 2017, our research group explored the modification of filter paper by simple dip coating in order to synthesize sorptive phases for analytical sample preparation (15). Our previous experience of recycled polystyrene (PS) was applied for this purpose (16). The proposed fabrication procedure consists of the previous dissolution of the polymer into an appropriate solvent and the dipping of the paper in the resulting solution a defined number of times. After each dip, the paper is dried, and the evaporation of the solvent leaves a film of the polymer over the paper. Figure 1(a) shows the complete procedure for the fabrication of PS paper. Note that in this case, PS is taken from yogurt containers, increasing the green character of the synthesis as contaminant plastics can be recycled.
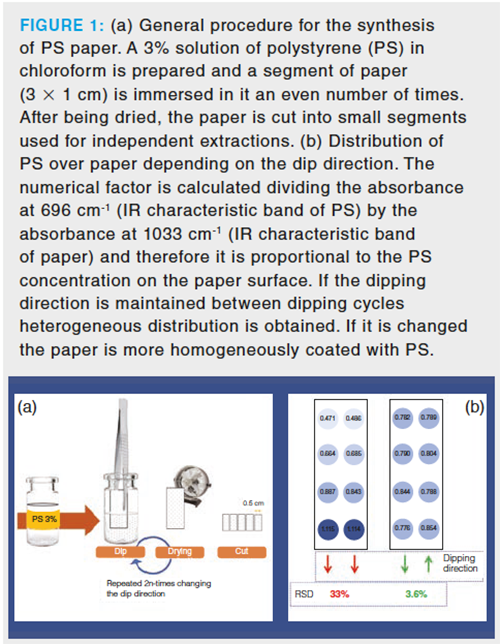
In this initial study, the dip direction was identified as a critical aspect to guarantee the homogeneity of the coating. A quantitative factor, measured by infrared (IR) spectroscopy and proportional to the PS amount, was used to monitor the coating homogeneity. If the dip direction is maintained in several consecutive dips, the polymers tend to accumulate in the lower part of the paper (see Figure 1[b]) providing a nonâhomogeneous coating (the relative standard deviation [RSD] of the factor was higher than 30%). However, if the dip direction is changed between dips and the number of immersions is even, the polymer is more homogeneously distributed in the paper (see Figure 1[b]) and the RSD is below 4%. This aspect is particularly critical because, as can be seen in Figure 1(a), the paper is finally cut into smaller segments that are used for independent extractions. Therefore, the homogeneity in the synthesis defines the extraction reproducibility.
The characterization of these materials was performed by IR spectroscopy and scanning electron microscopy (SEM). As way of example, Figure 2 shows the micrograph of a PS paper where a film of the polymer over the cellulose fibres is observed. The small holes over the surface can be ascribed to the evaporation of the solvent, and they can increase the porosity of the supports.

PS coating, as a result of the chemical characteristics, is useful for the extraction of nonpolar analytes with aromatic rings in their structure. These interactions are not selective at all, and therefore a chromatographic separation is needed for the individual determination of the extracted analytes. The selectivity can be improved further if mass spectrometry (MS) is used as the instrumental technique.
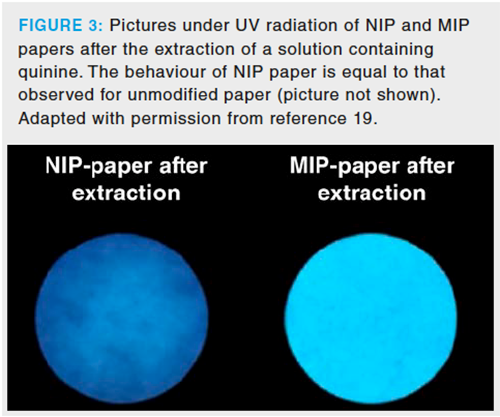
In a recent article, the group faced selectivity issues in the design of paper-based sorptive phases. Molecularly imprinted polymers (MIPs) present a network where matching cavities (in shape, size, and chemical environment) to the analyte or structure-related compounds are found. MIPs usually require a complicated procedure to be synthesized. In fact, the immobilization of classical MIPs over paper following a dip coating approach is not feasible because it requires the dissolution of the polymer, thus inducing the breakdown of the polymeric network and therefore of the selective cavities. Inspired by some articles describing the potential use of polyamides for the synthesis of MIPs (17,18), the direct polymerizationâfree synthesis of a MIP over filter paper was proposed (19). In this procedure, the polymer (nylon-6) is already present at the beginning of the synthesis. In this sense, nylon-6 is dissolved in formic acid, and the analyte is added to this solution. This solution is deposited on the paper, and the polymeric chains are arranged around the molecules of the analytes when the solvent evaporates, thus creating the selective cavities. These cavities present a chemically rich environment as polyamides can establish nonpolar (polymer backbone) and polar (amide group) interactions with the analytes. The enhanced selectivity of the MIP paper means that the chromatographic separation in the determination of quinine, which was selected as the proofâofâconcept analyte, can be avoided. The inherent fluorescence of the analyte, which also provides an additional selectivity enhancement, has been exploited to develop a luminescent sensor. Figure 3 shows two pictures where the selectivity of MIP paper is demonstrated. These pictures were acquired under UV light after the incubation of NIP and MIP paper with a standard solution containing the fluorescent analyte. After the incubation, the MIP paper provided a fluorescence signal more than four times higher than the NIP. MIP paper can also be applied in chromatographic analysis to obtain cleaner extracts, which could contribute greatly to an enhanced selectivity. The removal of intereferents would also permit the development of faster separations and would protect some elements (injectors, column, or instrument) from deterioration.
Recently, the modification of paper with polymeric ionic liquids synthesized by the Radziszewski reaction has been evaluated (20). The synthesis only requires the incubation of the paper with the polymer and a thermal curing, thus reducing the solvent requirements. The sorptive phase was evaluated for the extraction of some anti-inflammatory drugs from biofluids (urine) and the analytes were finally determined by liquid chromatography with tandem mass spectrometry.
Nanoparticles-Coated Paper
Nanoparticles (NPs) are materials that present special properties derived from their nanometric (<100 nm) size. They have been successfully applied in sample preparation because of their high surface area, which enhances both the thermodynamics and kinetics of the extraction. The types of existing NPs, their possible derivatization, and some additional properties (for example, the magnetism of magnetic NPs) makes these materials even more attractive.
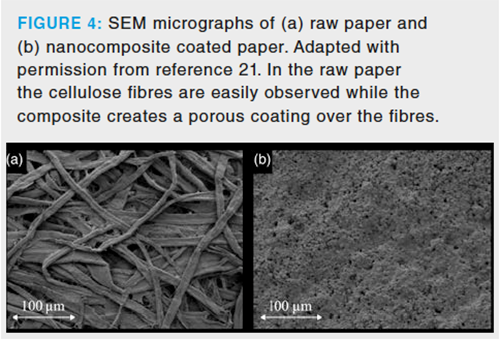
The immobilization of NPs in a paper by dip coating is challenging because the mechanical stability of the resulting film is compromised, and the coating can be removed during sample processing. This shortcoming can be overcome if the NPs are embedded in a polymeric network that protects them, thereby avoiding their release. Following this strategy, a polymeric composite comprising nylon-6 and TiO2 NPs was successfully immobilized over the paper (21). The synthetic procedure was similar to that previously described and consisted of the dissolution of nylon in formic acid and the subsequent dispersion of the NPs into the solution. Finally, the paper was dipped into the dispersion, and the evaporation of the solvent created a film of polymer with embedded NPs. Figure 4 shows the SEM micrographs obtained for raw paper and the nanocomposite-coated paper, where it can be seen how the cellulose fibres are entirely covered with the nanocomposite. Comparing Figure 4(b) and Figure 2 shows that the presence of NPs changes the morphology of the coating. The NPs are intercalated between the polymer chains disturbing their normal stacking, creating globular structures. This intercalation also increases the active surface of the polymer, thus boosting its sorption capability.
This nanocomposite-based paper efficiently combined the sorption capacity of nylon-6 and the photocatalytic activity of TiO2 NPs. In this sense, when the paper is incubated with an aqueous medium containing organic pollutants, these compounds are transferred to the sorptive phase (the polymer) as it occurs in SPME. The yield of this extraction depends on the chemical affinity of the pollutant–polymer. If a nanocomposite-based paper containing the extracted pollutant is irradiated with UV light, TiO2 NPs absorb this radiation creating a redox pair (electron–vacant) that degrades the contaminant. Although its application goes beyond the analytical sample preparation, it paves the road to future developments combining polymers and NPs.
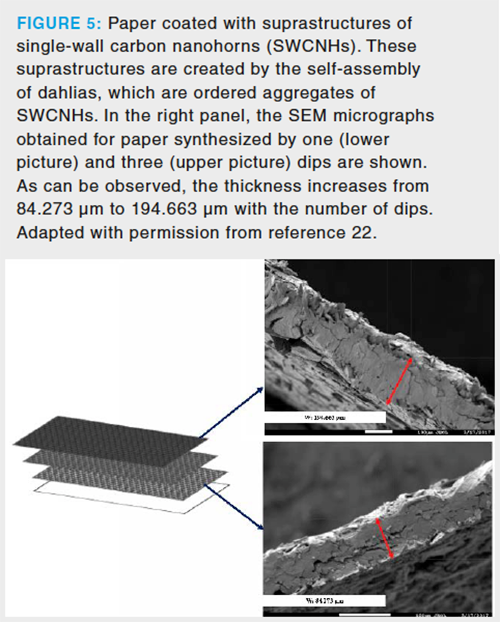
Although the use of a protective polymeric network is an elegant and efficient strategy to immobilize NPs over paper, the coating of paper with just pure single-wall carbon nanohorns (SWCNHs) was explored in 2018 (22). These nanoparticles present a tendency to create ordered aggregates (called dahlia) that assemble creating suprastructures of enhanced stability. If the paper is dipped
in a chlorformic dispersion of carbon nanohorns, the evaporation of the solvent leaves a pure SWCNHs coating whose thickness depends on the number of dips, as indicated in Figure 5. The surprising mechanical stability of this coating was ascribed to the extraction unit, and the entrapment of the first layer of the suprastructures into the pores of the paper. In fact, after the washing step, no visible loss of NPs were observed.
Extraction Units
Two different extraction units have been used for the practical application of these phases. In the first approach, a commercial plastic pipette tip was used to develop the full extraction, including the isolation and elution step. If the paper is cut to an appropriate size, it can be introduced in the tip and fixed by pressure in its narrower section (see Figure 6). Once located there, the phase is mechanically stable and different solutions can be aspirated and ejected efficiently using the pipette. The disposable nature of this extraction unit makes it attractive for bioanalysis, avoiding cross-contamination between samples and reducing the exposure of the analyst to biological risks. However, as a result of the geometry of the tip, not all the aspirated sample come into close contact with the paper-based sorptive phase (located on the walls of the tip). This means it is necessary to perform many aspiration–ejection cycles to isolate the compound from the sample with a high extraction recovery. Therefore, the use of pipette tips can be tedious if the extraction is done manually, but these devices are compatible with already existing autosamplers for pipette tip extraction.

Faster extractions can be achieved using inâvial extraction formats. In this example, the paper-based sorptive phase is added to the vial containing the sample and vortexed to enhance the transference of the analyte from the bulk sample to the sorptive phase. Several samples can be vortexed simultaneously, thus increasing the sample throughput. However, the sorptive phase must be manually collected after the extraction and transferred to an empty vial where the elution takes place.
Conclusions
This article gives a general overview of the synthesis of paper-based sorptive phases by dip coating. These phases can be used in the analytical sample preparation step to isolate (and preconcentrate) the target analytes from the sample matrix, thus improving the selectivity and sensitivity of the final chromatographic analysis.
The versatility of the coatings, which can be selected according to the analytical problem to be solved (binomial analyte/sample matrix), is especially relevant in this context. In addition, the simple synthesis opens the door to the transference of this approach to other laboratories. Up to now, polymers, NPs, and the combination of both have been evaluated as potential coatings with interesting results. Considering the wide variety of the primary components (polymers and NPs), there is a considerable number of combinations that are worthy of being evaluated.
The modification of paper by dip coating is a simple but effective strategy to fabricate sorptive phases. In fact, the synthetic procedure avoids complex steps (for example, reflux) and it uses a low quantity of reagents and solvents.
As has been demonstrated, paper-based sorptive phases can be applied not only in the resolution of different analytical problems but also in other fields like environmental remediation.
In the near future, research will be focused on three main lines: a) the development of new phases with enhanced selectivity; b) the evaluation of new extraction strategies (for example, flow-through) to increase the extraction recovery; and c) the direct coupling of these phases with instrumental techniques (for instance, MS) to speed up the analysis of samples.
Acknowledgements
The research topic presented in this article has been the core of the doctoral thesis of Julia Ríos-Gómez, who is thankful for the predoctoral grant (ref FPU13/03549) from the Spanish Ministry of Education. The financial support of the Spanish Ministry of Economy and Competitiveness through the grant CTQ2017-83175R made this research possible. This project was started by the authors of the article, but other researchers of the group have also been involved in developments. We want to thank their efforts and contributions over these two years: Dr. Ángela I. López-Lorente, Dr. Beatriz Fresco-Cala, Dr. M. Teresa García-Valverde, Beatriz Ferrer-Monteagudo (B.Sc.), and M. Carmen Díaz-Liñán (B.Sc.). We would like to thank Prof. Luque for his contribution to the photocatalytic paper development. This article is based upon work from the Sample Preparation Task Force and Network, supported by the Division of Analytical Chemistry of the European Chemical Society.
References
- D.E. Raynie, LCGC Europe32(3), 144–147 (2019).
- J. Zheng et al., TrAC Trends Anal. Chem. 108, 135–153 (2018).
- M.N. Alam, E. NazdrajiÄ, V. Singh, M. Tascon, and J. Pawliszyn, Anal. Chem. 90(19), 11548–11555 (2018).
- H. Piri-Moghadam, M.N. Alam, and J. Pawliszyn, Anal. Chim. Acta984, 42–65 (2017).
- I. Bruheim, X. Liu, and J. Pawliszyn, Anal. Chem. 75(4), 1002–1010 (2003).
- Y.A. Olcer, M. Tascon, A.E. Eroglu, and E. Boyacı, TrAC Trends Anal. Chem.113, 93–101 (2019).
- E.M. Reyes-Gallardo, R. Lucena, and S. Cárdenas, TrAC - Trends Anal. Chem.84, 3–11 (2016).
- E. Zilfidou, A. Kabir, K. Furton, and V. Samanidou, Separations5(3), 40 (2018).
- V. Kazantzi and A. Anthemidis, Separations4(2), 20 (2017)
- M.C. Alcudia-León et al., J. Chromatogr. A1488, 17–25 (2017).
- X. Meng, Q. Liu, and Y. Ding, Electrophoresis38(3–4), 494–500 (2017).
- M. Saraji and B. Farajmand, J. Chromatogr. A1314, 24–30 (2013).
- A.H. Ackerman and R.J. Hurtubise, Anal. Chim. Acta474(1–2), 77–89 (2002).
- E.-A. Kim and Y.Y. Lim, Microchem. J.145, 979–987 (2019).
- J. Ríos-Gómez, R. Lucena, and S. Cárdenas, Microchem. J. 133, 90–95 (2017).
- H. Ghambari, E.M. Reyes-Gallardo, R. Lucena, M. Saraji, and S. Cárdenas, Talanta170, 451–456 (2017).
- X. Zhu and Q. Zhu, J. Appl. Polym. Sci.109(4), 2665–2670 (2008).
- E.V. Dmitrienko et al., J. Mol. Recognit.26(8), 368–375 (2013).
- M.C. Díaz-Liñán, A.I. López-Lorente, S. Cárdenas, and R. Lucena, Sensors Actuators B Chem.287, 138–146 (2019).
- J. Ríos-Gómez et al., Anal. Chim. Acta1094, 47–56 (2020).
- J. Ríos-Gómez et al., J. Clean. Prod.194, 167–173 (2018).
- J. Ríos-Gómez, B. Fresco-Cala, M.T. García-Valverde, R. Lucena, and S. Cárdenas, Molecules23(6), 1252 (2018).
Julia Ríos-Gomez received a B.Sc. in environmental sciences from the University of Cordoba, Spain, in 2013. During that year, she was a collaborator student at the Analytical Chemistry Department at the University of Cordoba and completed her M.Sc. in chemistry in 2014, where she studied the potential of the hybrid material microparticle-aptamer to improve the selectivity of protein identification. She received her Ph.D. degree in November 2018.
Rafael Lucena is a professor of analytical chemistry at the Department of Analytical Chemistry and secretary of the Institute of Nanochemistry of the University of Cordoba (Spain). His main research interests on the sample treatment context are related to the development of new extraction techniques, the application of new solvents, and the development of new sorbents.
Soledad Cárdenas is a full professor of analytical chemistry at the University of Cordoba where she also served as vice chancellor for academic affairs and competitiveness. Her research interest is focused on the development of new materials (for example, monolithic columns) in the sample treatment context. Her research group has made several contributions to the development of new extraction techniques. She is the coordinator of the Spanish Network on Sample Treatment, which is funded by the Spanish Ministry of Innovation, Science, and Universities.

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)





