Size-Exclusion Chromatography for the Analysis of Complex and Novel Biotherapeutic Products
LCGC Europe
This study presents applications of size-exclusion chromatography (SEC) for characterization and quality control of novel biotherapeutic products, including antibody–drug conjugates, hydrophobic proteins, and coformulations. Examples of modifying SEC mobile-phase composition and running conditions to modulate the separation are discussed, as well as approaches and strategies for analyzing atypical protein products such as coformulations.
Recombinant biotherapeutics have been produced and marketed for several decades, providing life-changing medicines for a variety of indications. With the maturation of the biotherapeutic market over recent years, novel protein products such as conjugates, bispecifics, fusion proteins, and coformulations are being developed. The detailed characterization and quality control of complex biopharmaceuticals has proven to be more challenging than the typical two-light-chain/two-heavy-chain monoclonal antibody products. This study presents applications of size-exclusion chromatography (SEC) for characterization and quality control of novel biotherapeutic products, including antibody–drug conjugates, hydrophobic proteins, and coformulations. Examples of modifying SEC mobile-phase composition and running conditions to modulate the separation are discussed, as well as approaches and strategies for analyzing atypical protein products such as coformulations.
Modern biological drug development stems mostly from using recombinant DNA technology in living microorganisms such as Escherichia coli or Chinese hamster ovary (CHO) cells to produce therapeutic agents (1). These recombinant biotherapeutics have been produced and marketed for several decades, providing lifeâchanging medicines for a variety of indications (2). With the maturation of the biotherapeutic market over recent years, novel protein products are now being developed, including antibody–drug conjugates (ADCs) (3), bispecific antibodies (4), and coformulations (5). Although detailed characterization and quality control of biopharmaceuticals are expected for regulatory approval, the physicochemical analysis of novel biotherapeutics with their more complex formats is proving to be more challenging than the more traditional protein products, such as typical twoâlightâchain/two-heavy-chain recombinant monoclonal antibodies (mAbs).
There are several categories of complex biotherapeutics, and only a few are described in this article. Twoâlightâchain/two-heavy-chain mAbs are commonly developed as therapeutics because of their specificity to a chosen biological target. ADCs leverage this specificity by having small-molecule drugs attached to a mAb, such that when the mAb binds to a specific target cell, the entire complex is internalized and subsequently releases its small-molecule drug payload into the target cells (3). This approach reduces the chance of off-target side effects because the potent small-molecule drug is mostly released into target cells. Bispecific mAbs, in turn, simultaneously bind to two targets as a result of different amino acid sequences in each Fab arm-for example, different complementarity determining regions (CDRs) in each antigen-binding fragment (Fab) arm-whereas standard mAbs have the same CDRs in each Fab arm (4). Coformulations are mixtures of two or more different active pharmaceutical ingredients (APIs) in the same drug product, thus allowing for simultaneous dosing of multiple drugs and offering both increased convenience to the patient and potential synergistic effects (5). Other complex biotherapeutic formats, such as Fc-fusion proteins, are in clinical development, but are not addressed in this article.
This report covers several applications using size-exclusion chromatography (SEC) to characterize challenging or complex mAb products. SEC is used to separate high-molecular-weight forms (HMW) or aggregates from the main peak, which primarily contains the monomeric protein species. To achieve size-based separations, SEC columns are packed with porous material that serves as the stationary phase. In the mobile phase, molecules of various sizes flow into the column, and smaller dissolved molecules flow more slowly through the column because they penetrate into the pores of the stationary phase, whereas large molecules flow more quickly through the column because they are more excluded from the pores and are eluted in the void volume (6). Consequently, larger molecules are eluted from the column earlier and smaller molecules are eluted later, effectively separating the molecules by hydrodynamic radius, which generally correlates to molecular weight for mAbs.
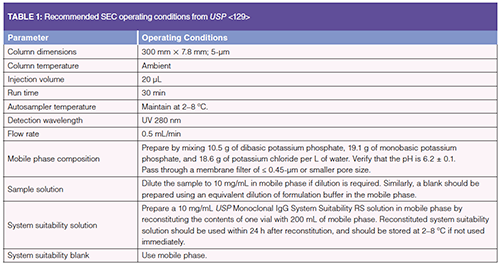
Size exclusion–high performance liquid chromatography (SE-HPLC) has been the gold standard for size variant analysis for decades (6). SEâHPLC is routinely used in quality control (QC) and development laboratories to quantify protein aggregates, which are of particular concern to health authorities because of their potential to elicit an immunogenic response (7,8). Aggregates and protein fragments may also affect the potency and pharmacokinetics (PK) of the drug product (9). A generic SEC method has been published in the U.S. Pharmacopeia (USP) General Chapter <129>, “Analytical Procedures for Recombinant Therapeutic Monoclonal Antibodies”, and the recommended conditions are shown in Table 1. A major advance in the field of SEC has been the development of size exclusion–ultrahighâpressure liquid chromatography (SE-UHPLC), which utilizes instrumentation featuring decreased system volume and higher allowable back pressures compared to typical HPLC instruments. SE-UHPLC has been demonstrated for highâthroughput, high-resolution applications (10), and has been validated for use in QC laboratories (11). Figure 1 demonstrates the improvements in speed and resolution of a monoclonal antibody (mAb1) separated by SE-UHPLC relative to SE-HPLC. HMW forms are eluted before the main peak and low-molecularâweight forms (LMW) are eluted after the main peak. Note that peak integration strategy may vary from product to product (for example, continuous flat baseline versus continuous valley-toâvalley baseline versus discontinuous peak-to-peak baseline), but different integration strategies may be acceptable as long as they are adequately justified (for example, a slanted baseline may cause a discontinuous baseline to be more accurate than a continuous flat baseline). In Figure 1, note that the SEâUHPLC fragment peak after the main peak is grouped with the main peak. This grouping is done to obtain similar relative peak area quantification with the SEâHPLC method, which does not resolve this fragment peak from the main peak.
Although the SE-HPLC method in USP Chapter <129> is suitable for many mAbs, the examples in this article will demonstrate that the USP <129> method may not be optimal for every biotherapeutic product. In these cases, a variety of strategies may be employed to improve the SEC separation, such as changing the mobile phase composition or switching from the HPLC version (the USP <129> method) to the UHPLC version to increase the resolution between size variant peaks. Detailed method development and troubleshooting of SEC methods are not covered in this article; rather, this article focuses on various complex and novel protein products (ADCs, hydrophobic proteins, bispecific antibodies, and coformulations) that benefit from advanced understanding of SEC separations and improved column and instrument technologies to enable better resolution and improved quantitation of protein size variants.
Materials and Methods
Columns and Chemicals: SE-HPLC experiments were performed using a Tosoh TSKgel G3000SWXL column, 7.8 mm × 300 mm, 5-µm. SE-UHPLC experiments were performed using either a Waters Acquity BEH200 SEC column, 4.6 mm × 300 mm, 1.7âµm, or a Tosoh TSKgel UP-SW3000 column, 4.6 mm × 300 mm, 2-µm.
Monoclonal antibodies were produced in-house at Genentech. The thermally stressed sample for mAb3 was produced by incubating mAb3 at 40 °C for 14 d. The oxidation stressed sample for mAb3 was produced by incubating mAb3 with 1.6 mM AAPH (2,2’-azobis(2-amidinopropane) dihydrochloride) for 24 h at 40⯰C. The light-stressed sample for mAb3 was produced by subjecting mAb3 to 1.36 million lux hours (M lux h) for a duration of 24 h. The thermally stressed sample for mAb5 was produced by incubating mAb5 at 40 °C for four weeks. Common buffers, salts and solvents were procured from Fisher Scientific or VWR.
Equipment: SE-HPLC chromatographic experiments were performed on either an Agilent 1100/1200/1260 HPLC instrument or a Waters 2695(e) HPLC system. SE-UHPLC chromatographic experiments were performed on either a Waters Acquity H-Class UPLC or a ThermoScientific UltiMate 3000 RSLC system. Components of the system included a high-pressure gradient binary pump (ThermoScientific RSLC) or a low-pressure gradient quaternary pump (Waters UPLC), a column compartment capable of temperature control, an autosampler with sample temperature control capability, and a tunable UV–vis detector. Instrument control, data acquisition, and compilation of results were performed using ThermoScientific Chromeleon software.
Methods: SE-HPLC was performed using the SEC method conditions published in the USP Chapter <129>, “Analytical Procedures for Recombinant Therapeutic Monoclonal Antibodies”, (Table 1), unless otherwise indicated. SE-UHPLC was performed using the SE-UHPLC method conditions published by Graf and associates (11), unless otherwise indicated.
mAb samples were diluted with mobile phase and kept at a temperature of 5 °C ± 3 °C in the autosampler. The column effluent was monitored at 280 nm. A blank injection was performed with each sequence prior to sample injection. After the installation of a new column, conditioning runs were performed until consistent profiles were achieved. Each chromatogram was carefully integrated to ensure that only peaks not present in the associated blank were considered to be protein (ThermoScientific Chromeleon software).
ADCs and Hydrophobic Proteins
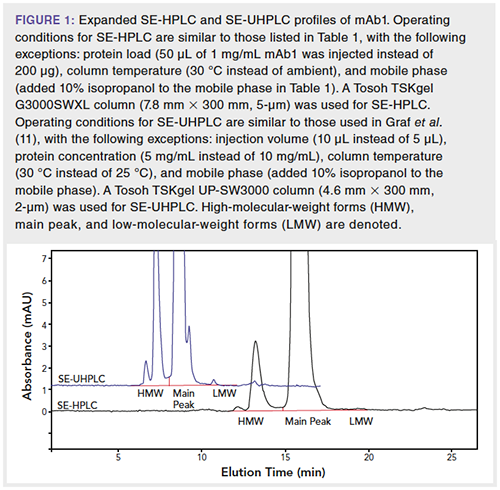
Although SEC serves as a primarily size-based separation method, SEC has also been demonstrated to separate species based on hydrophobicity due to the interaction of the analytes with the stationary phase (12). These secondary interactions sometimes result in undesired increases in elution time and peak tailing, which may be mitigated by mobile phase additives. For example, the addition of organic modifiers has been shown to reduce hydrophobic interactions between the protein analytes and the stationary phase (13). Figure 2 shows a hydrophobic mAb analyzed by SE-HPLC with and without the use of an organic modifier (15% isopropanol). The addition of the organic modifier decreases the main peak elution time and alters the peak profile. It should also be noted that the main peak width decreased with the addition of organic solvent to the mobile phase. Therefore, organic modifier in the mobile phase may be explored if peak broadening or peak tailing is significant for the main peak.
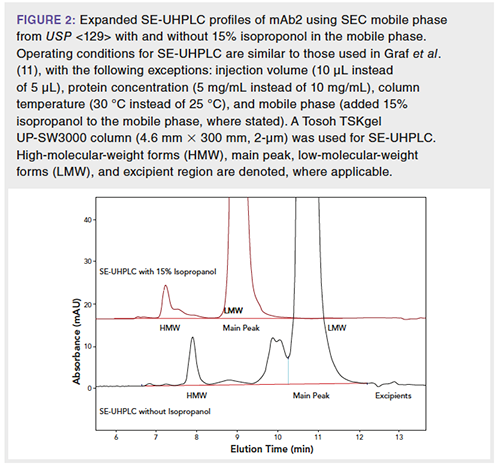
ADCs generally have increased hydrophobicity compared to a standard mAb as a result of the hydrophobic linker drugs attached to the mAb. Thus, an SE-UHPLC method with 10% isopropanol in the mobile phase was developed to mitigate the hydrophobic interactions between the ADC and the stationary phase (Figure 3). Upon different degradative stresses, the relative peak area of the HMW forms increase compared to the control. Peaks representing higher order aggregates (in other words, larger than dimer) increase significantly upon different degradative stress conditions and elute earlier than the main HMW forms (dimer).
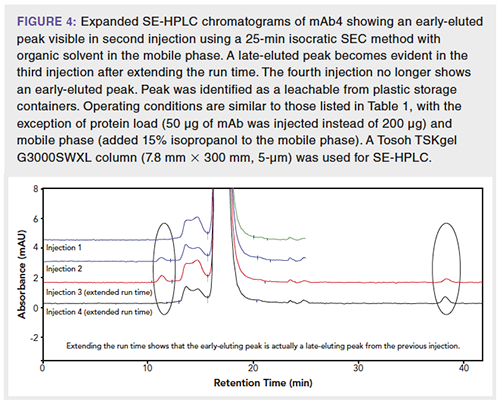
While the use of an organic modifier in the SEC mobile phase can be helpful for improving resolution for hydrophobic analytes, addition of organic solvent may cause leachables from plastic containers to be eluted off the column and subsequently appear in the SEC chromatograms as new peaks. Given that leachables are small, they are eluted well after the analytes of interest, and a method that is too short would cause the leachables to appear in subsequent runs (Figure 4). In this instance, further characterization determined that the peak was a leachable generated from prolonged storage of sample in polypropylene tubes, and that organic solvent in the mobile phase caused the leachable to be eluted off the column. Indeed, organic solvents such as isopropanol are also used as extraction solvents during extractable studies (14). Therefore, careful consideration of sample container materials may be needed to avoid new, undesired peaks resulting from leachables.
Bispecific Antibodies
As described previously, bispecific antibodies are designed to simultaneously bind to two different epitope targets due to the different amino acid sequences in each Fab arm. In some cases, one Fab arm of the bispecific antibody may be longer than the other Fab arm (11). Unlike standard mAbs, bispecific antibody products have unique but undesirable product variants such as homodimer (both Fab arms bind to the same target), mispaired or scrambled light chain, and single arm half-antibodies (15). Some of these variants may be resolved using SEC, with SE-UHPLC resulting in better resolution of these bispecific variants compared to SE-HPLC (11).
In one study, SEC directly coupled to native mass spectrometry (SEC-MS) was developed to rapidly characterize a bispecific antibody and its variants (15). Generally, SEC methods do not have sufficient resolving power to resolve size variants of similar masses; however coupling MS to native SEC (SEC-MS) can be used to identify noncovalent and covalent size variants with similar elution times. This tool is of particular importance for bispecific antibodies because bispecific aggregates and fragments often have similar SEC elution times as their homologous homodimer variants, thus requiring an orthogonal technique (in this case, MS) to identify the peaks of interest.
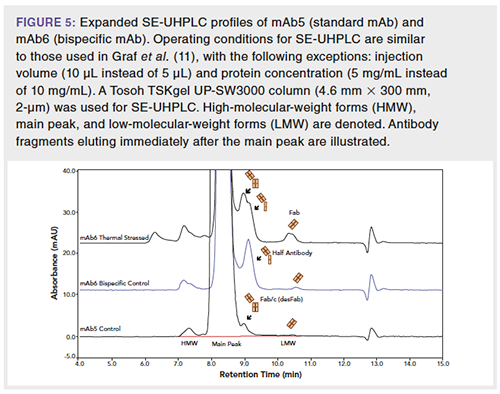
Because bispecific molecules have different types of size variants than traditional mAbs as a result of their asymmetrical structure (two different antibody halves in one mAb), their SEC profiles tend to have different peak profiles than traditional mAbs. The arrows in Figure 5 show fragment peaks eluted at different times for the standard mAb and the bispecific mAb. These differences result from the fact that the fragment in the mAb profile is Fab/c (otherwise known as a desFab or one-armed antibody), whereas the fragment in the bispecific mAb profile is primarily half antibody, which has a lower molecular weight and later elution time than the Fab/c fragment. Thermally stressing the bispecific mAb produces more Fab/c fragment, and the Fab/c and half-antibody peaks in the stressed sample profile can be partially resolved by SE-UHPLC (Figure 5).
Coformulation of Multiple Protein Products
With an increasingly diverse selection of mAb products in development and on the market, combination therapies of multiple biotherapeutics are being developed, further leveraging the wide selection of biotherapeutics available to treat complex diseases. Combination mAb therapies can be sequentially administered at the clinic (one drug after another) or coâadministered (two drugs in the same IV bag). For increased patient convenience, some manufacturers are moving towards coformulated drug products where two or more biotherapeutics are combined in the same vial and released by the manufacturer for shipment to the clinic (5). While the coformulated drug product is more convenient for the patient, it can be challenging for the manufacturer to develop analytical methods and specifications to ensure acceptable product quality of each individual drug in the comixed product (16).
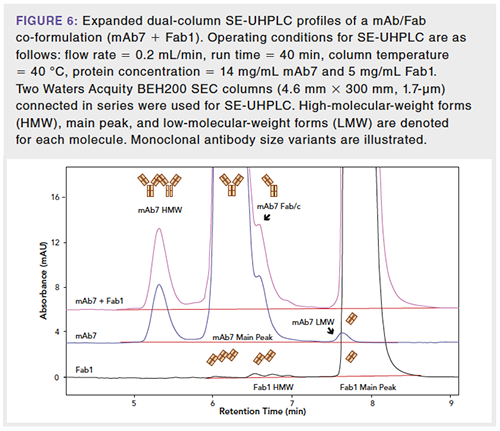
A typical SEC method, such as the USP <129> SE-HPLC method, can be used to analyze coformulated or comixed biotherapeutics (16). However, the resolution of the USP <129> method may not be adequate for product quality assessment in a coformulation, particularly for two drugs having similar molecular weights. In these cases, various strategies may be employed to further increase the resolution to enable quantitation of the size variants in the coformulated drug product. For instance, an analyst may switch to SE-UHPLC or multiple SE-UHPLC columns in series to further improve the separation (17). Figure 6 shows the chromatograms obtained from a mAb + Fab (antibody fragment) coformulation using dual-column or tandem SE-UHPLC (for example, two columns connected in series). Although not all peaks are fully resolved, tandem SE-UHPLC provides better resolution than single-column SE-HPLC. For degraded mAb + Fab samples (not shown), it may be difficult to determine by SEC which product variant from either mAb7 or Fab1 has increased in the overlapping peak regions. SEC-MS (15) or SEC with multi-angle light scattering (SEC-MALS) (16) can be employed to elucidate the identities of the product variants in degraded coformulation samples, and SEC-MS may provide relative quantities of the product variants in the overlapping peak regions. With this information, the likely degradation pathway of the coformulated product may be predicted, and the tandem SE-UHPLC method may be used to monitor the size variants of the coformulated product based on the predicted degradation pathway. In other words, although the tandem SE-UHPLC method may not resolve all size variants, the improved resolution and knowledge of the degradation pathway may be leveraged to interpret results from accelerated stress studies or real-time stability studies.
Discussion and Conclusion
This article presents examples of SEC analysis of novel biotherapeutic products, which are generally more complex than traditional mAbs. Specifically, we covered hydrophobic molecules, ADCs, bispecific mAbs, and coformulations, all of which have unique considerations when developing and performing SEC methods. For hydrophobic molecules and ADCs, we demonstrated that adding organic modifier in the mobile phase reduced elution time of the main peak because of the disruption of the hydrophobic interaction between the protein and the stationary phase. We also showed that the addition of organic modifier can alter the peak pattern of the SEC separation. Therefore, one must use caution when adding organic solvent to the mobile phase, as unwanted changes to the peak pattern may occur in addition to the desired improvements in elution time and resolution.
Bispecific molecules and coformulations generally have more size variants than standard mAbs. Bispecific molecules may have unwanted halfâantibody in the sample that SEC can resolve from Fab/c (desFab) and Fab. Coformulated drug product samples have roughly twice as many product variants as a single API drug product. Because bispecific antibodies and coformulations have more variants to resolve, improved resolution in SEC analyses may be required for thorough physicochemical characterization of the product. In addition to the strategies presented here, other techniques, such as multidimensional LC (18), may be developed to achieve improved peak separation.
The rapid development of biotherapeutics has resulted in increasingly complex products, including ADCs, bispecific mAbs, and coformulations. Size-exclusion chromatography techniques have also improved to allow for better resolution, faster run times, and improved characterization of these analytically challenging formats. Here we presented examples of SEC approaches for the analysis of ADCs, hydrophobic mAbs, bispecific mAbs, and coformulations. These examples demonstrate that although a generic SEC method has been published in the pharmacopeia, additional SEC method development may be required for the analysis of complex biotherapeutics.
Acknowledgements
The authors would like to acknowledge Armando Cordoba for his technical input, Adithi Bhargava and Lucy Li in Pharmaceutical Development for generating stressed samples, David Michels and Matt Kalo for reviewing this manuscript, and the global Roche/Genentech SEC Analytical Expert Team (AET) for fruitful discussions and collaboration.
References
- J.M. Reichert, C.J. Rosensweig, L.B. Faden, and M.C. Dewitz, Nature Biotech.23(9), 1073–1078 (2005).
- A. Philippidis, Genetic Engineering and Biotechnology News (11 March 2019).
- N. Diamantis and U. Banerji, Br. J. Cancer114(4), 362–367 (2016).
- S.E. Sedykh, V.V. Prinz, V.N. Buneva, and G.A. Nevinsky, Drug Des. Devel. Ther.12, 195–208 (2018).
- M. Cao, N. De Mel, A. Shannon, M. Prophet, C. Wang, W. Xu., B. Niu, J. Kim, M. Albarghouthi, D. Liu, E. Meinke, S. Lin, X. Wang, and J. Wang, mAbs11(3), 489–499 (2019).
- P. Hong, S. Koza, and E.S.P. Bouvier, J. Liq. Chromatogr. Rel. Technol.35, 2923–2950 (2012).
- T. Uchino, Y. Miyazaki, T. Yamazaki, and Y. Kagawa, J. Pharm. Pharmacol.69(10), 1341–1351 (2017).
- W. Jiskoot, G. Kijanka, T.W. Randolph, J.F. Carpenter, A.V. Koulov, H.-C. Mahler, M.K. Joubert, V. Jawa, and L.O. Narhi, J. Pharm. Sci.105, 1567–1575 (2016).
- J. Vlasak and R. Ionescu, mAbs3, 253–263 (2011).
- E.S.P. Bouvier and S.M. Koza, Trends in Anal. Chem. 63, 85–94 (2014).
- T. Graf, R. Ruppert, A. Knaupp, G. Hafenmair, S., Violini, and S. Kiessig, LCGC North Amer.36(12), 870–879 (2018).
- J.A. Pavon, X. Li, S. Chico, U. Kishnani, S. Soundararajan, J. Cheung, H. Li, D. Richardson, M. Shameem, and X. Yang, J. Chromatogr. A1431, 154–165 (2016).
- T. Arakawa, D. Ejima, T. Li, and J.S. Philo, J. Pharm. Sci.99, 1674–1692 (2010).
- D.L. Norwood, D. Paskiet, M. Ruberto, T. Feinberg, A. Schroeder, G. Poochikian, Q. Wang, T.J. Deng, F. DeGrazio, M.K. Munos, and L.M. Nagao, Pharm. Res. 25(4), 727–739 (2008).
- M. Haberger, M. Leiss, A.K. Heidenreich, O. Pester, G. Hafenmair, M. Hook, L. Bonnington, H. Wegele, M. Haindl, D. Reusch, and P. Bulau, mAbs8, 331–339 (2016).
- Z.W.K. Glover, L. Gennaro, S. Yadav, B. Demeule, P.Y. Wong, and A. Sreedhara, J. Pharm. Sci.102(3), 794–812 (2013).
- S. Fekete, A. Beck, J.-L. Veuthey, and D. Guillarme, J. Pharm. Biomed. Anal.101, 161–173 (2014).
- A. Ehkirch, A. Goyon, O. Hernandez-Alba, F. Rouviere, V. D’Atri, C. Dreyfus, J.-F. Haeuw, H. Diemer, A. Beck, S. Heinisch, D. Guillarme, and S. Cianferani, Anal. Chem.90, 13929–13937 (2018).
Jennifer Rea, David Fulchiron, Yun Lou, and Luda Darer are with Genentech, in South San Francisco, California, USA.

Polysorbate Analysis in Biopharmaceutical Applications — A Snapshot of the Analytical Toolbox
An overview of different approaches for the qualitative and quantitative analysis of polysorbates.
The LCGC Blog, Paying it Forward: Perspectives from a Fulbright–Palacky Distinguished Scholar
January 5th 2024In this LCGC Blog, Kevin Schug shares his plans for the future as a Fulbright–Palacky University Distinguished Scholar in the Czech Republic, and discusses why it is important to share analytical chemistry with communities around the world.
What Are Employers Looking For When Hiring Separation Scientists?
August 1st 2023LCGC North America spoke to Caroline McGregor and Justin Pennington of Merck Research Laboratories regarding what employers are looking for in candidates they are considering for open separation scientist positions.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)





