Next-Gen Sorbent-Based Extractions with Metal-Organic Frameworks
Metal-organic frameworks (MOFs) are coordination networks consisting of a metal ion linked with organic ligands. The resulting three-dimensional structures create pores that can be exploited for a number of chemical processes, including analytical extractions. The resulting sorbent-based extraction systems have several advantages, notably selectivity. The use of MOF in extraction has exploded in the last two or three years. In this article, we take a look at the current state of the art regarding analytical extractions utilizing MOF, including a description of what MOF are, their preparation, principles of use, advantages, and application areas.
The field of sample preparation and analytical extractions faces a somewhat unique contradiction. On one hand, analysts are seeking high levels of selectivity during these preliminary stages. That is, there is a desire to isolate our analytes of interest to the exclusion of everything else prior to the actual analysis. On the other hand, advances in chromatography, mass spectrometry, and spectroscopy over the past couple of decades have allowed us to characterize samples of increasing impurity. Significant increases in gaining selectivity during the sample preparation steps of an analysis are gained with the use of selective adsorbents. These extractions include solid-phase extraction (SPE), solid-phase microextraction (SPME), stir-bar sorptive extraction (SBSE), dispersive extractions (including QuEChERS, matrix-solid phase dispersion [MSPD], and dispersive SPE [dSPE]), and a host of similar techniques related to sorbent-based methods. Our last sample preparation trends survey, conducted five years ago (1), demonstrated steadily increasing use of these techniques. Historically, these sorbent-based extractions featured use of either chromatography stationary phases or general sorbents, like silica, carbon, or alumina. More recently, specialized sorbents are being used for sorbent-based extractions, such as molecularly imprinted polymers, restricted access media, crown ethers, and others.
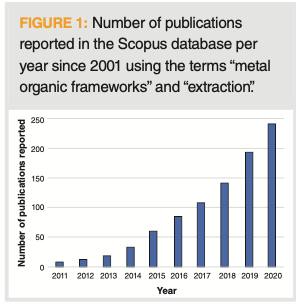
Last year at this time (2), we reported on the major sample preparation advances in the previous year. One major advance, still in an emerging state, was the use of metal organic frameworks (MOFs) for extraction. This was highlighted by one of our thought leaders and featured prominently in an Analytical Chemistry fundamental review (3). During this past year, the use of MOFs in analytical extractions has exploded from the emerging state to the break-through phase. We can see in Figure 1 an almost exponential growth in the annual number of publications, as reported with the Scopus database using the search terms “metal organic frameworks” and “extraction,” over the past decade. In 2020, nearly 250 articles were reported, compared with just 8 in 2011! With this explosive growth, there must be some distinguishing features surrounding the use of metal organic frameworks (MOFs) for extraction. In this “Sample Prep Perspectives” installment, we explore this phenomenon, with a focus on the past year or two.
Metal Organic Frameworks
MOFs are coordination polymers or highly ordered crystalline structures, typically two- or three-dimensional, composed of metal cations or clusters connected with coordinating organic ligands (4,5). They are mesoporous with pores in the 2–50 micron diameter range. MOFs can have a surface area in the thousands of m2/g with a high number of pores and functional groups. Self-assembly of the metallic moieties with multifunctional organic ligands containing nitrogen and/ or oxygen comprise the MOF. The first permanently porous MOF were reported in 1995 (6). The coordination between the metallic component and the organic ligand is described by the hard/soft acid/base (HSAB) theory. Because of the chemical nature of the MOFs, intermolecular forces, including electrostatic interactions, Van der Waals forces, hydrophobic interactions, π–π interactions ion exchange, Lewis acid-base, chelation, hydrogen bonding, and coordination can adsorb analytes from various mixtures during an extractive procedure (7–9).
Synthesis of MOF can be by a variety of routes, including slow evaporation, covalent assembly, chemical co-precipitation hydrothermal, solvothermal, microwave-assisted method, mechanochemical, and electrochemical techniques (10,11). Because of these synthetic procedures, there is wide latitude in creating MOFs with crystalline properties, tunable porosity, and surface areas in the range of 2000–7000 m2/g. Additionally, variable pore volumes can be created, and uniform porous structures achieved, with high thermal and mechanical stability (8). MOFs functionalized with ionic liquids are even being produced (12). Given the number of available functional groups and metal ions or clusters available, it is conceivable that the potential number of available MOFs to be created is infinite.
Extraction Modes
Essentially all modes of sorbent-based extractions have been performed with MOFs, including the in-tube and in-syringe approaches to SPE. This is because of their key features. MOF sorbents have high tunable porosity and surface area, designable structures, internal functionalities, and outer surfaces available for molecular interactions. MOFs also have thermal and mechanical stability, structural cavities, and uniform active sites (13,14). The MOFs can be included as part of polymer matrices, or in the pores of organic monoliths. As a result of these properties, MOFs have been used in conventional and dispersive SPE, though compaction and flow irregularities with cartridge SPE seem to lead to the dispersive approach being favored. With dispersive SPE, the MOFs are rather easily dispersed with the sample matrix and recovery of the MOFs via phase separation is often straightforward. SPME and SBSE are also popular approaches for using MOFs during analytical extractions. One unique opportunity of MOFs is via magnetization of the metal component. After mixing the MOFs, frequently as nanoparticles, with the sample, recovery can be quite simple. Analyte enhancement factors using MOFs is large, often in the thousands. Extraction efficiencies with MOFs are similar to other sorbents and the extraction configuration; that is, the ratio of MOF to sample amount and analyte concentration, sample volumes, surface area, identify and volume of sample and eluent solvents, flow rates, extraction times, ionic strength, and sample phase can all play important roles in extraction efficacy, as they would in conventional and dispersive SPE, SPME, or SBSE. Thus, the selectivity, solvent use, recovery, and other advantages of these techniques still hold.
Applications
Given all of the stated advantages of MOFs and their explosive growth in the literature, one can expect that application of MOFs in sorbent-based extractions are manifold. These applications are found with both liquid and solid samples and, while not exclusive, are found primarily in the biological, environmental, and food areas. Table I summarizes applications found in recent reviews (5,8,10,11,13,15–17).
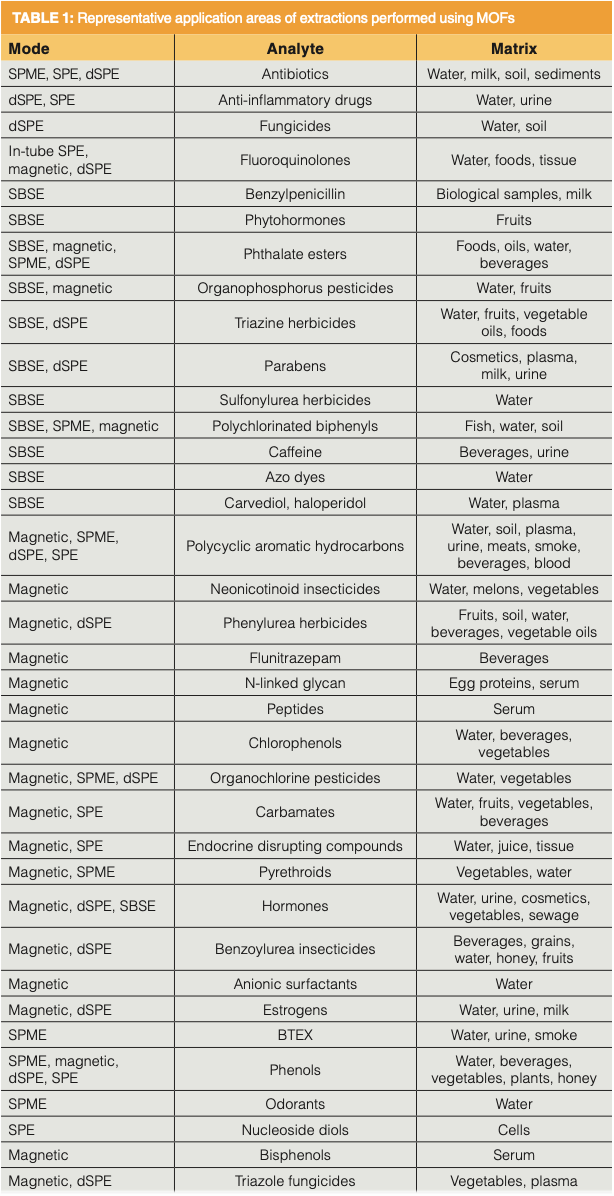
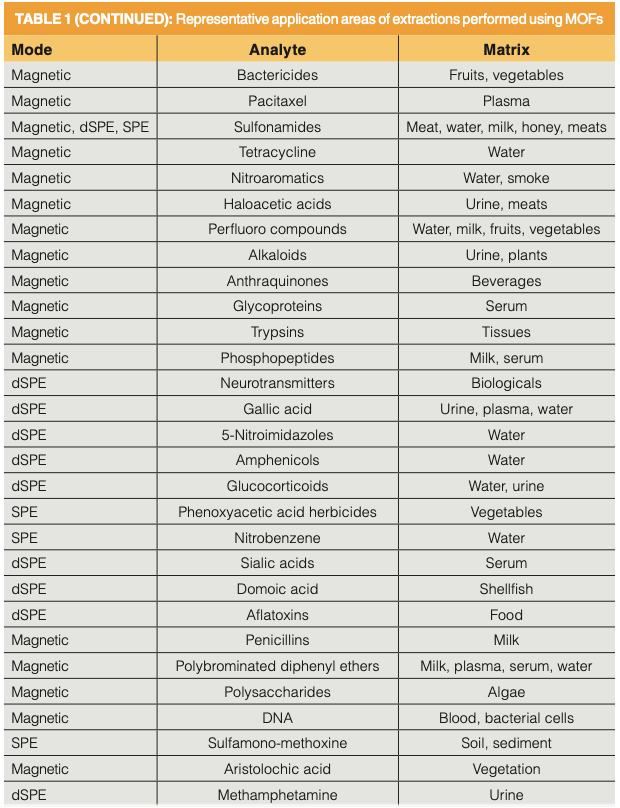
A few key observations are gleaned from this table. Conventional SPE, SPME, and SBSE techniques using MOFs are somewhat evenly distributed, yet are the more minor approaches to MOF extractions. It is the approaches that take special advantage of the unique properties of MOFs that make up the majority of the applications. These approaches are dSPE and use of magnetic MOF. In the biological field, drugs of interest included antibiotics, nonsteroidal anti-inflammatories, penicillins, and methamphetamine in urine, milk, and tissues. Additional biomolecules isolated from these matrices include estrogens and hormones, carbohydrates, peptides, proteins, and aflatoxins. Each of the major environmental contaminants of recent interest are extracted with MOF, namely endocrine disruptors, most types of herbicides and pesticides, phthalate esters, parabens, polychlorinated biphenyls and polybrominated diphenyl ethers, polycyclic aromatic hydrocarbons, polyfluorinated compounds in environmental waters and wastewater, soils and sediments, body fluids, fruits and vegetables, and related sample types. Fruits, vegetables, beverages, edible oils, and meats were investigated for herbicides and pesticides, hormones, and aflatoxins.
Conclusions and Future Prospects
An emerging type of sorbent material, MOFs, is presented for use in conventional and dispersive SPE, SPME, SBSE, and, especially, magnetic extractions. These MOFs are characterized by high surface area, controlled and tunable porosity, high stability, and significant functionalization. Applications to biological, environmental, and food samples abound. Analytical extraction with MOFs as a field is only about a decade old and growing rapidly. Consequently, continued use of MOFs for new application areas, including industry standard and regulatory methods, are the obvious growth area. Currently, there are no commercial MOF extraction materials and consumables available; such commercialization will help drive these additional applications.
References
(1) D.E. Raynie, LCGC N. Am. 34(3), 174–188 (2016).
(2) D.E. Raynie, LCGC N. Am. 38(3), 159–162 (2020).
(3) F.A. Hanson and S. Pedersen-Bjergaard, Anal. Chem. 92, 2–15 (2020).
(4) M. Ghorbani, M. Aghamohammad- hassan, H. Ghorbani, and A. Zabihi, Microchem. J. 158, 105150 (2020).
(5) J.C. Masini, F.H. do Nascimento, and R. Vitek, Trend Environment. Anal. Chem. 29, e0112 (2021).
(6) O.M. Yaghi and G.M. Li, Angewandte Chemie–Int. Ed. Engl. 34, 207–209 (1995).
(7) S.C. Vardali, N. Manousi, M. Barczak, and D.A. Giannakudakis, Molecules 25(3), 513 (2020).
(8) M. He, Y. Wang, Q. Zhang, L. Zhang, B. Hin, and B. Hu, J. Chromatogr. A 1637, 461810 (2021).
(9) C.K. Hasan, A. Ghiasvand, T.W. Lewis, P.N. Nesterenko, and B. Paull, Anal. Chim. Acta 1139, 222–240 (2020).
(10) R. Seetharaji, P. Vandana, P. Arya, and S. Mathew, Arab. J. Chem. 12, 295–325 (2019).
(11) Q. Wang, T. Gao, L. Hao, Y. Guo, W. Liu, L. Guo, C. Wang, Z. Wang, and Q. Wu, TrAC Trends Anal. Chem. 132, 116048 (2020).
(12) S. Wan, O. Xu, and X. Zhu, Ionics 27(2), 445–456 (2021).
(13) M. Sajid, M.K. Nazal, and I. Ihsanullah, Anal. Chim. Acta 1141, 246–262 (2021).
(14) H. Duo, X. Lu, S. Wang and Y. Guo, TrAC Trends Anal. Chem. 133, 116093 (2020).
(15) A. Gutierrez-Serpa, P.I. Napolitano-Tabares, J. Sulc, I. Pacheco-Fermamdez, and V. Pino, Separations 7(3), 1–32 (2020).
(16) T. Rasheed, M. Bilal, A.A. Hassan, F. Nabeel, R.N. Bharagava, L.F. Roman- holo Ferreira, H.N. Tran, and H.M.N. Iqbal, Environ. Res. 185, 109436 (2020).
(17) L. Li, Y, Chen, L. Yang, Z. Wang, and H. Liu, Coord. Chem. Rev. 411, 212235 (2020).
COLUMN EDITOR
“Sample Prep Perspectives” editor Douglas E. Raynie is a Department Head and Associate Professor at South Dakota State University, USA. His research interests include green chemistry, alternative solvents, sample preparation, high-resolution chromatography, and bioprocessing in supercritical fluids. He earned his PhD in 1990 at Brigham Young University, Utah, USA, under the direction of Milton L. Lee. Raynie is a member of LCGC’s editorial advisory board. Direct correspondence to: amatheson@mjhlifesciences.com.

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)




