Multi-Residue Analysis of Pesticides in Fruits Using DisQuE, a Dispersive Solid-Phase Extraction Kit
A new technique, QuEChERS, standing for Quick, Easy, Cheap, Effective, Rugged, and Safe, is readily accepted by both the AOAC International and the Committee of European Normalization (CEN) for the pesticide residues in foods and agriculture products. Waters DisQuEâ„¢ Dispersive Sample Preparation Kit contains conveniently-packaged centrifuge tubes with pre-weighed sorbents and buffers designed for use with the AOAC official QuEChERS methods.
Jeremy C. Shia, Michael S. Young, and Diane M. Diehl, Waters Corporation
A new technique, QuEChERS, standing for Quick, Easy, Cheap, Effective, Rugged, and Safe, is readily accepted by both the AOAC International and the Committee of European Normalization (CEN) for the pesticide residues in foods and agriculture products. Waters DisQuE™ Dispersive Sample Preparation Kit contains conveniently-packaged centrifuge tubes with pre-weighed sorbents and buffers designed for use with the AOAC official QuEChERS methods.
The DisQuE Dispersive Sample Preparation Kit contains: DisQuE extraction (tube 1), a 50 mL centrifuge tube containing 6 g of anhydrous magnesium sulfate and 1.5 g of anhydrous sodium acetate, and DisQuE clean-up (tube 2), a 2 mL centrifuge tube containing 50 mg of primary secondary amine (PSA) sorbent and 150 mg of anhydrous magnesium sulfate.
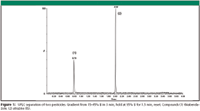
Figure 1
The typical procedure consists of two steps: a well homogenized aqueous sample is first extracted with acetonitrile in the presence of anhydrous magnesium sulfate and acetic acid/sodium acetate. After centrifugation the organic extract is further cleaned up by dispersive solid-phase extraction (d-SPE), typically using primary secondary amine (PSA) sorbent. The PSA sorbent, combined with MgSO4, in tube 2 is very effective for removing organic acids, excessive water, and other components. The extract is subsequently analyzed by either GC or LC (or both), often coupled with mass spectrometry (MS) depending on the pesticides of interest.
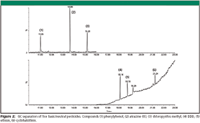
Figure 2
Sample Preparation
The fruit sample is chopped into small portions, and then pulverized by a food blender until it reaches homogeneous texture. Transfer 15 g of the homogenized fruit sample into the extraction tube 1, and then add 15 mL of 1% acetic acid in acetonitrile. Shake the tube vigorously for 1 min and centrifuge at 1500 rcf for 1 min. Transfer 1 mL of the upper layer extract from tube 1 to clean-up tube 2. Shake tube 2 vigorously for 1 min and centrifuge at 1500 rcf for at least 1 min. The extract is subsequently analysed by either GC–MS or LC–MS depending on the pesticides of interest.
Results and Discussion
Pesticides were fortified into two fruit matrices: grape and avocado. Atrazine was used as the internal standard (IS) for both GC–MS and LC–MS analysis. A spiking solution of the target pesticides was prepared at 50 μg/mL of each compound. 40 μL of the spiking solution was added to 10 mL of sample extract to give a nominal concentration of 200 ng/mL for each pesticide. The same amount of pesticides was spiked to the extracts either prior to the SPE procedure or after the SPE cleanup. The recovery of each pesticide was calculated by comparing the concentrations in the samples where the pesticides were spiked prior to the SPE procedure to those where the pesticides were spiked after the SPE cleanup. The recovery results in avocado and grape are summarized in Table I. The % recoveries of pesticides in grape are ranged from 97% to 109%. The pesticides are recovered in avocado ranging from 96% to 110%. There was no significant loss of pesticides due to the SPE clean-up procedure using PSA sorbent.

Table I: The % recovery results of pesticides fortified in grape and avocado. The % recovery is mean value of triplicate sample analysis.
Conclusions
The DisQuE dispersive sample preparation kit is convenient to use. The simple and straightforward procedure is very effective for removing matrix interferences commonly associated with fruit matrices without significant losses of pesticides. This procedure is applicable to most basic and neutral pesticides. Acidic pesticides can be analyzed directly from Tube 1 using a suitable dilution buffer.

Waters Corporation
34 Maple Street, Milford, MA 01757
tel. (508) 478-2000, fax (508) 478-1990

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)
















