Fast Gradient LC Using Your Existing Instrumentation
The Application Notebook
Transforming "standard" gradient HPLC systems into extremely fast gradient systems is readily achievable with proper application of chromatographic principles, particularly temperature control, combined with utilization of advanced HPLC columns. Bottom line: You don't have to buy a new LC to achieve ultra high quality and speed!
Chris Cantelmo1, Jody Clark2, Qing P. Han3, and Mark J. Hayward3, 1GL Sciences, Inc., 2Selerity Technologies, and 3Lundbeck Research
Transforming "standard" gradient HPLC systems into extremely fast gradient systems is readily achievable with proper application of chromatographic principles, particularly temperature control, combined with utilization of advanced HPLC columns. Bottom line: You don't have to buy a new LC to achieve ultra high quality and speed!
The quest to pursue "ultra" fast LC separations continues to gain momentum as the result of recent product introductions to address this need. These new products claim to deliver the same separations while using 5–10 fold less time. What may be lesser known is that routine performance of "ultra" fast separations can be achieved using many of the ordinary main stream HPLCs that one may already have available. Achieving this level of performance is simply a matter of knowledge of the separation principles involved and their practical implementation. A key principle involved is balancing flow with molecular velocity to and from the stationary phase surface (mass transfer). The implementation of the LC set up involves volume control in the system and its eluted peaks. The implementation of the LC analyses involves making good choices in stationary and mobile phases as well as full proper control of critical system parameters such as mobile phase temperature (entering column to control mass transfer).
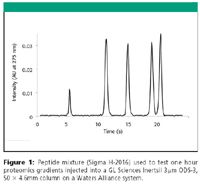
Figure 1
Application of the above concepts allows routine analyses of >500 samples per day per LC (or LC–MS) operating at 7 mm/s (5 mL/min, 5× "normal"), clearing ~9 column volumes (k') per min (~10x "normal") and delivering peaks <1 s wide, all using ordinary HPLC systems that are 2–20 years old. One result of this added capacity is the straightforward and scalable positive impact on turnaround times of existing analyses while conserving resolution (quality). In our normal workflow, we routinely demonstrate that it is possible to sustain rates of >5000 quantitative LC (or LC–MS) analyses per instrument per person per month.
Experimental Conditions
The authors have demonstrated gradient chromatograms like the ones shown here in >106 analyses using a wide variety of instrumentation from Agilent, Shimadzu, and Waters. The data shown here comes from a Waters Alliance 2795 with standard column oven or a Waters 1525 pump with CTC auto-sampler and Analytical Sales Hot Sleeve column heater. In either case, the detector was a Waters 2996 PDA operated at 20 Hz. The column used was a 50 × 4.6 mm Inertsil HPLC column packed with 3 micron ODS-3 provided by GL Sciences, Inc. (P/N 1010L050W046), which offers lower back pressure than most columns operating under similar conditions. Separation temperature was 60°C and controlled primarily with a CaloraTherm (Selerity Technologies) active eluent pre-heater to provide the proper energy needed to maintain a constant temperature as the heat capacity changes with the fast gradient.
Results
Fast separations require use of increased eluent velocities. The higher one can increase the velocity, the more times one can sweep the column per unit time and thus more resolution that one can achieve per unit time. A crucially important step in optimizing at these higher velocities involves temperature control to achieve the required (optimum) mass transfer rate (1–3). Use of ordinary ovens alone (air contact) is insufficient to achieve constant temperature across the column because the energy exchange rate to the eluent is too large and variable due to gradient (4, 5). The recent availability of active on-line (no adding volume or cross section) eluent pre-heaters solves this problem and allows routine use of broad gradients with real-time DT <2°C between column inlet and outlet. Then, temperature can be optimized allowing ultra fast separations to be performed on ordinary HPLCs as shown in Figure 1 where the peptide mix normally used to test 1 h proteomic gradients is fully resolved in 21 s. It is very desirable to maintain relatively constant temperature across the column during fast acetonitrile gradient operation. Finally having control of temperature, allows one to take full advantage of today's best of the best high performance reverse phase columns with any HPLC and routinely produce very fast separations, even for classically challenging basic compounds, such as shown in Figure 2.

Figure 2
It is important to note that the ultimate minimum separation efficiency of reverse phase LC systems is largely defined by the injection process (volume delivered to column), (6, 7) not the particle diameter. In an optimized system, the column may be contributing as little as 20% of the observed peak width (variance) (7) and at that point there is very little more that can be done to improve the separation (narrow the peaks) using particle diameter reduction. Therefore, the known diminishing of the benefit of smaller particles (8–11) must be weighed against the rapidly increasing pressures (inversely proportional to particle diameter squared) that will require reduction in velocity to stay within available pressure limits. The best of the best stationary phases in ~3 micron diameters have been empirically shown to provide both best separation efficiency and highest velocities (fastest analyses) (12). Smaller particles yield lower velocities (due to high pressures) and larger particles (>5 micron) yield lower separation efficiency due to lost (slower) mass transfer. The 3 micron Inertsil particles are noteworthy because they consistently yield lower pressures and performance that is equivalent to that of smaller diameter ones including the sub 2 micron choices. The lower pressure extends the range of higher velocities that can be accessed with ordinary LC systems beyond that which is currently commonly practiced with new ultra high pressure systems using sub 2 micron particles. These even faster separations are clearly demonstrated in Figures 1 and 2. The crucial combination of moderate pressure along with quality bonding and ultra high purity silica used in these particles minimizes secondary adsorption interactions and allows optimized operation where the separation efficiency is clearly not particularly limited by the column.
Conclusions
Applying an understanding of the critical operating parameters of a gradient HPLC separation with quality GL Sciences' Inertsil columns and Selerity's unique CaloraTherm active eluent heating system allows modern ultra fast results to be achieved in a very cost effective way using traditional gradient HPLC systems. The performance shown is achieved on a routine basis, has been reproducibly repeated >106 times, and the technique employed can be implemented on virtually any HPLC system.
Please contact GL Sciences, Inc. at (310) 265-4424 or info@inertsil.com to find out more about achieving the speeds shown here using your LC. GL Sciences can provide step-by-step instructions on how to optimize your HPLC for ultra fast analyses (in PDF format). Also inquire about the complete ultra fast optimization kit that is available for your HPLC.
References
(1) F.D. Antia, Cs. Horvath, J. Chromatogr. 1988, 435, 1.
(2) B. Yan, J. Zhao, J.S. Brown, J. Blackwell, P.W. Carr, Anal. Chem. 2000, 72, 1253.
(3) F. Gritti, A. Felinger, G. Guiochon, Anal. Chem. 2006, 78, 5329.
(4) R.J. Perchalski, B.J. Wilder, Anal. Chem. 1979, 51, 774.
(5) R.G. Wolcott, J. W. Dolan, L.R. Snyder, S.R. Bakalyar, M.A. Arnold, J.A. Nichols, J. Chromatogr. A 2000, 869, 211.
(6) L.R. Synder & J.J. Kirkland, "Introduction to Modern Liquid Chromatography," John Wiley & Sons: New York, 1974.
(7) F. Gritti, A. Felinger, G. Guiochon, J. Chromatogr. A 2006, 1136, 57.
(8) M.J. Wirth, J. Chromatogr. A 2007, 1148, 128.
(9) A.D. Jerkovich, J.S. Mellors, J.W. Jorgenson, LCGC 2003, 21, 600.
(10) A. de Villiers, H. Lauer, R. Szucs, S. Goodall; P. Sandra, J. Chromatogr. A 2006, 1127, 60.
(11) L.A. Colon, J.M. Cintron, J,A. Anspach, A.M. Fermier, K.A. Swinney, Analyst 2004, 129, 503.
(12) T.L. Chester, S.O. Terami, J. Chromatogr. A 2005, 1096, 16.

GL Sciences, Inc. USA
4733 Torrance Blvd., Ste 255, Torrance, CA 90503
tel. (310) 265-4424, fax (310) 265-4425

The Benefits of Custom Bonded Silica
April 1st 2025Not all chromatography resins are created equal. Off-the-shelf chromatography resins might not always meet the rigorous purification requirements of biopharmaceutical manufacturing. Custom bonded silica from Grace can address a wide range of separation challenges, leading to real performance improvements. Discover more about the latest innovations in chromatography silica from Grace, including VYDAC® and DAVISIL®.
5 Things to Consider When Selecting a Chromatography Silica
April 1st 2025Particularly in the pharmaceutical industry, drug purity isn’t just a goal – it’s essential for achieving safety, stability and efficacy. However, purification is easier said than done, especially with challenging molecules like DNA and RNA “oligonucleotides,” due in large part to their diversity and the range of impurities that can be generated during production. Enter DAVISIL® chromatographic silica, with a wide range of pore diameters and particle sizes to meet your specific application, performance and sustainability requirements. Before you choose the chromatography resin for your next purification application, take a look at these 5 considerations.
Automating Protein Purification: Efficiency, Yield, and Reproducibility
March 27th 2025Recent advancements in automated protein purification stress the importance of efficiency, scalability, and yield consistency. This eBook compares different purification platforms, highlighting their impact on downstream applications and demonstrating how automation enhances throughput and process control.
MilliporeSigma: Ultrapure Water for Sensitive LC-MS Analysis of Pesticides
March 25th 2025The aim of the study was to illustrate the efficiency of Milli-Q® water purification systems in eliminating pesticides from tap water, thereby producing and delivering reliable and consistent-quality ultrapure water suitable for pesticides analysis















