Monitoring of On-column Methionine Oxidation as Part of a System Suitability Test During UHPLC–MS/MS Peptide Mapping
Special Issues
Long-term column use can lead to on-column methionine oxidation during LC–MS/MS peptide mapping of antibody-based biotherapeutics. Following the approach described here minimizes the risk of measuring oxidative artifacts, and helps generate high quality data to provide reliable quantitative information about product-related heterogeneities.
Peptide mapping by liquid chromatography–tandem mass spectrometry (LC–MS/MS) is an essential analytical tool for the characterization of biotherapeutics, such as for the identification and quantification of post-translational modifications, including oxidation. Therefore, is it crucial that the LC–MS system is performing under optimal conditions, ensuring reproducible and accurate quantitative information. A system suitability test is usually performed prior to sample analysis to confirm that the sample preparation and instrument performance are adequate and running as expected. Induced in-source oxidation is a known phenomenon associated with the electrospray process, and can be discriminated from relevant sample oxidation. On-column methionine oxidation can also take place during LC–MS/MS peptide mapping analysis of antibody-based biotherapeutics over long-term column use. This article describes how the monitoring of on-column methionine oxidation as part of system suitability testing can be used to control unintended on-column methionine oxidation of biotherapeutics. This approach minimizes the risk of measuring oxidative artifacts, and helps generate high quality data to provide reliable quantitative information about product-related heterogeneities.
Björn Mautz*, Vincent Larraillet*, Maximiliane König, and Michael Mølhøj
* Both authors contributed equally to this work.
Peptide mapping by ultrahigh- pressure liquid chromatography–tandem mass spectrometry (UHPLC–MS/MS) is extensively used for various purposes, such as identity testing, disulfide bond analysis, host cell protein analysis, sequence variant analysis, assessment of potential critical quality attributes, and multiple-attribute methodology (MAM) workflows for biotherapeutics, including the evaluation of post-translation modifications (PTMs). PTMs occur at distinct amino acid side chains or peptide linkages, and are added shortly after translation is completed, after folding during passage through the Golgi apparatus, upon bioprocessing, storage, stress, or after administration. In therapeutic proteins, PTMs are especially critical if they negatively influence drug potency or safety. It is therefore essential to ensure reproducibility in the manufacturing process of therapeutic proteins so that there is no significant difference in the effective dose, and to prevent unexpected side effects. Consequently, PTMs need to be monitored and controlled to demonstrate batch consistency and comparability of manufactured clinical material. It is essential that the sample preparation method, the liquid chromatography system, and the mass spectrometer are performing optimally to ensure reliable data. For example, suitable endoproteolytic digestion methods at mildly acidic conditions (pH 6.0) have been developed to keep to a minimum artificial deamidation and succinimide formation (1–2).
Oxidation is a source of protein variability and one of the major degradation pathways for protein therapeutics in vitro and in vivo. The sulfur-containing cysteine (unpaired) and methionine residues are particularly susceptible to oxidation (3), and are often monitored during formulation development, batch characterization, and long-term stability studies. Protein oxidation can lead to diverse functional consequences, such as lower activity or potency, increased susceptibility to aggregation, and altered immunogenicity (4–10). Methionine residues are readily oxidized to methionine sulfoxide (MetO) S- and R-diastereomers by many reactive species adding oxygen to the sulfur atom (11–13), and the reaction is essentially pH independent (14). Given that MetO is more hydrophilic relative to methionine, it will elute earlier on a reversed-phase column (15). The level of sample oxidation can be easily quantified by comparing the peak area of the MetO with the methionine peak. In addition to the sample oxidation, a redox reaction in the electrospray ionization (ESI) source can also occur, causing artificial in-source oxidations (16–17). Usually, in-source oxidation can be easily detected based on the identical elution profile of modified and unmodified peptides, allowing in-source oxidation to easily be discriminated from in-sample oxidation (15). MetO can be further oxidized to methionine sulfone (MetO2). Other commonly oxidized residues include tryptophan and histidine (18–19).
A system suitability test (SST) is typically part of an analytical method to confirm that the sample preparation and the entire instrument performance are adequate to ensure reproducible and accurate results (20-21). Lack of an adequate SST may generate poor quality data and misleading information. Here, we report the observation of unexpected on-column methionine oxidation during LC–MS/MS peptide mapping analysis and its impact on the SST sample. This study shows how the quantitative evaluation of methionine oxidation was found to be susceptible to significant variability over long-term reversed-phase column use, and how a suitable SST may mitigate the effects of on-column oxidation.
Materials and Methods
Enzymes and Antibodies
Trypsin was purchased from Promega. The bispecific antibody (bsAb1) sample was stably expressed in Chinese hamster ovary (CHO) cells, and purified from a platform fed batch fermentation. The SST sample consisting of a monoclonal antibody (mAb1, lambda light chain) spiked with 0.5% (w/w) of a second monoclonal antibody (mAb2, kappa light chain) was stored as ready-to-use 25 μL aliquots (concentration is 10 mg/mL) at –80 °C.
Tryptic Digests
Both bsAb1 and the SST sample were denatured and reduced in 0.3 M tris-HCl pH 8, 6 M guanidine-HCl and 20 mM dithiothreitol (DTT) at 37 °C for 1 h, and alkylated by adding 40 mM iodoacetic acid (13C: 99%) (Sigma-Aldrich) at room temperature and in the dark for 15 min. Excess iodoacetic acid was inactivated by adding DTT to a total of 40 mM. The alkylated proteins were buffer exchanged using NAP5 gel filtration columns (GE Healthcare), and digested with trypsin in 50 mM tris-HCl, pH 7.5 at 37° C for 16 h. The reactions were stopped by adding formic acid to 0.4% (v/v). Digested samples were stored at –80 °C until analysis.
UHPLC–MS/MS Analysis
The tryptic digests were analyzed by UHPLC–MS/MS using a Vanquish UHPLC system (Thermo Fisher Scientific) and an Orbitrap Fusion Lumosmass spectrometer (Thermo Fisher Scientific). For each analyzed sample, 2.5 µg of digested protein were injected onto the UHPLC system. Chromatographic separation was performed by reversed-phase LC on a BEH300 C18 column (1 mm x 150 mm, 1.7-µm) (Waters) using mobile phase A and B containing 0.1% formic acid (v/v) in UHPLC grade water and acetonitrile, respectively (Optima LC–MS quality; Fisher Chemical). A 60 µL/min flow rate, a 50 °C column temperature, and the following gradient were used: 1% mobile phase B (0–3 min), 1% to 40% mobile phase B (3–93 min), 40% to 99% mobile phase B (93-94 min), 99% mobile phase B (94–96 min), 99% to 1% mobile phase B (96–97 min), and 1% mobile phase B (97–105 min). Two blank injections of mobile phase A, using a 50 min gradient up to 99% mobile phase B, were performed between sample injections to reduce carry-over. The SST sample was run at the beginning and the end of each sequence to ensure suitability of the system for the entire sequence. The column was stored in 80% (v/v) acetonitrile after usage.
MS/MS Data Acquisition
High-resolution MS/MS spectra were acquired with the orbital trap mass analyzer, and detection of higher-energy collisional dissociation (HCD) fragment ion spectra with dynamic exclusion enabled (repeat count of 1, exclusion duration of 15 s [±10 ppm]). The orbital trap Fusion Lumos was used in the data-dependent mode. HCD essential settings were: full MS (automatic gain control (AGC): 4 x 105, resolution: 1.2 x 105, m/z range: 300-2000, maximum injection time: 50 ms), MS/MS OT (AGC: 5.0 x 104, maximum injection time: 500 ms, isolation window: 2), orbital trap resolution was 15 x 103, MS/MS IT (AGC: 1.0 x 104, maximum injection time: 100 ms, isolation window: 2), normalized collision energy was set to 28%.
Data Processing
Analysis of the MS/MS data was performed using the Byos/Byologic v3.3 software (Protein Metrics). Manual data interpretation and quantification was performed using the Xcalibur Qual Browser v.4.0 (Thermo Fisher Scientific). Extracted ion current chromatograms were generated with the most intense isotope masses.
Results and Discussion
During the peptide mapping analysis of the unstressed bsAb1 sample, unexpected high levels of oxidations were observed using a qualified UHPLC–MS/MS based method and a reversed-phase column that at the time of this analysis had been used for ~1500 column runs. The observed oxidation affected several methionine-containing tryptic peptides from complementarity-determining, variable framework, and the fragment crystallizable (Fc) regions. The six mostly oxidized peptides are listed in Table I. Relative oxidation levels ranging from 0.1% to 2% were expected for all oxidized peptides based on historical data. However, using the aged column, the oxidation levels of the six peptides ranged between 3% and 30% (Table I). When the same samples were reanalyzed on a new column under the same conditions, the observed oxidation levels were comparable to the expected values (historical data) for all oxidized peptides (Table I).
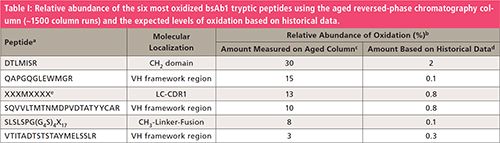
A closer inspection of the extracted ion current (EIC) chromatograms of the oxidized peptides using the aged column demonstrated that the chromatograms exhibited unusual elution profiles. Using the aged column, the oxidized peptides eluted continuously over ~5–9 min, giving rise to distorted and broadened peaks (Figure 1a–c, 1g–i). Using a new column, the oxidized peptides eluted as distinctive peaks (Figure 1d–f, 1j–l). Given that this kind of continuous elution profile for oxidized peptides (on the aged column) had not been observed previously, the initial hypothesis was that either the eluents or the chromatography column could be the potential root cause. Given that the same mobile phases were used with both the aged and new column runs, the broadened oxidation peaks could only be explained by an on-column oxidation reaction taking place on the stationary phase surface of the UHPLC column during the peptides’ elution. Using the new column, the in-sample and in-source induced oxidized peptides were baseline separated and typical profiles were observed in the course of the chromatographic separation (Figure 1d–f, 1j–l). In-sample oxidized peptides had earlier retention times than the in-source oxidized peptides due to the interaction with the reversed-phase column by the in-sample oxidized peptides. On-column oxidation was defined by oxidation that was only observed with the aged reversed-phase column. No increased methionine di-oxidation or tryptophan oxidation were observed in any bsAb1 peptides.
Figure 1: Extracted ion current chromatograms of unmodified (black) and oxidized (red) bsAb1 tryptic peptides (amino acid sequence indicated, see also Table I) using an aged (~1500 column runs) (a-c and g-i) and a new (d-f and j-l) reversed-phase C18 column. (c and f) The amino acid sequence of the LC-CDR1 covering peptide indicated by XXXMXXXX is undisclosed. (j) Two in-sample oxidation peaks observed likely due to the peptide (amino acid sequence: SQVVLTMTNMDPVDTATYYCAR) containing two methionine residues. In-Sa, in-sample oxidation; On-Co., on-column oxidation; In-So., in-source oxidation; URP, unrelated peptide.
The unusually high levels of peptide oxidation using the aged column compelled us have a deeper look at the SST data, and the system performance in general. The SST selected for this study was a sample consisting of a monoclonal antibody mAb1 spiked with 0.5% (w/w) of a second monoclonal antibody mAb2. The SST sample was digested with the same procedure and at the same time as the other samples, and then run at the beginning and at the end of each sequence. The sample preparation and LC–MS system are considered suitable if three acceptance criteria are met, namely 1) the mAb2 sequence coverage (positive identification by MS/MS of ≥70% of 20 unique peptides covering the complementarity-determining regions and the kappa light chain), 2) the mAb2 peptide recovery rate (≥15 of the 20 unique peptides), and 3) consistent retention times within acceptable ranges for three mAb2 tryptic peptides (A, B, and C) for all SST samples within a sequence. Further evaluations included visual inspections of the total ion current (TIC) chromatograms of the blank and the sample runs.
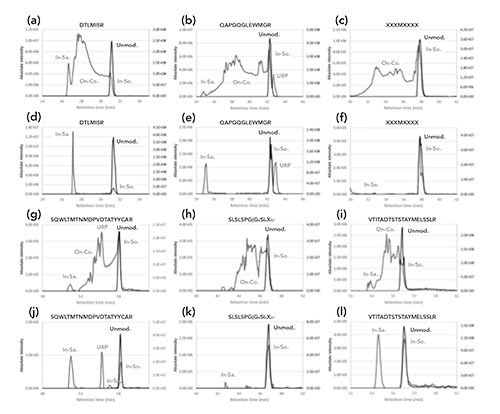
In the present study, the SST sample was used on both the aged and the new columns. Although the mAb2 sequence coverage and peptide recovery rate of the 20 unique peptides were slightly reduced on the aged column compared to the new column, all three SST criteria were met using the aged column (Table II). However, a visual inspection of the TIC chromatograms revealed some differences between the new and the aged columns in the intensities of a few peaks (peaks 1–7, Figure 2), which were attributed to the presence or absence of missed trypsin cleavages (Figure 2). These data suggested that the differences between the aged and new column TIC profiles could also be related to the sample preparation (tryptic digest efficiency) in addition to the column aging.

Figure 2: Total ion current chromatograms of the tryptic digested system suitability test sample (surrogate mAb1 spiked with 0.5% (w/w) mAb2), using (a) a new column (two injections), and (b) an aged reversed-phase C18 column. The apparent differences (numbered peaks) were identified as the following mAb1 peptides: 1) heavy chain AA349-359 without missed cleavages, 2) heavy chain AA349-364 with one missed cleavage, 3) heavy chain AA223-252 with two missed cleavages, 4) heavy chain AA306-321 without missed cleavages, 5) heavy chain AA227-252 with one missed cleavage (increased levels with the aged column) and heavy chain AA20-38 without missed cleavages (similar levels with both columns), 6) unassigned peptides likely containing missed cleavages, and 7) light chain AA131-150 without missed cleavages. NL, normalized intensity level.
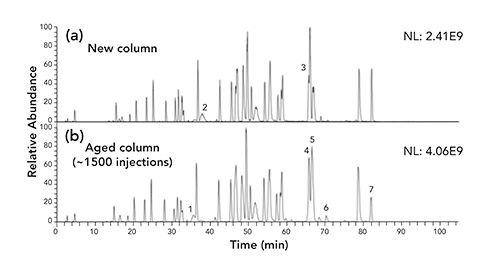
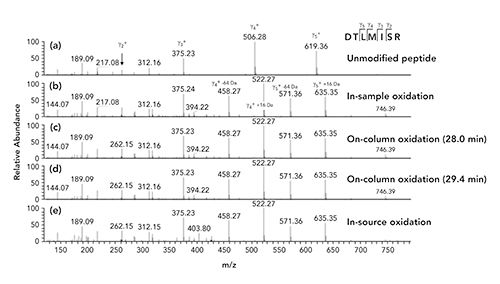
Using the aged column, the peptide with the amino acid sequence DTLMISR was determined to be the most oxidized amongst the tryptic bsAb1 peptides (Table I). This peptide is common to the Fc region of all four subclasses of immunoglobulins (IgG1-4), and the methionine residue Met252 is known to be susceptible to oxidation in intact immunoglobulins (22–23). Similarly to the bsAb1 sample, the DTLMISR peptide of the SST sample was also determined to be the most oxidized with approximately 5% in-sample, 24% on-column, and 1% in-source oxidation on the aged column (Figure 3a). As the oxidized DTLMISR peptides were not baseline separated, a more exact quantification is difficult with the aged column. Besides, the in-sample and in-source oxidation peaks may contain some on-column oxidation. Using the new column, the same peptide was determined to be 2% in-sample and 0.4% in-source oxidized, and no on-column oxidation was detected (Figure 3b). An inspection of the MS/MS spectra of the SST sample using the aged column confirmed that the in-sample (elution time: 26.7 min), on-column (at 28.0 min and 29.4 min), and in-source (31.0 min) modified peptides (Figure 3a) all contained an oxidized methionine residue in the same position (DTLMISR) (Figure 4).
Figure 3: Extracted ion current chromatograms of the single protonated, unmodified (black) and oxidized (red) system suitability test sample peptide DTLMISR using (a) the aged (~1500 column runs) reversed-phase C18 column, and (b) a new reversed-phase C18 column. For the aged column, approximate relative numbers are indicated as the oxidized peptides were not baseline separated. In-Sa, in-sample oxidation; On-Co., on-column oxidation; In-So., in-source oxidation.
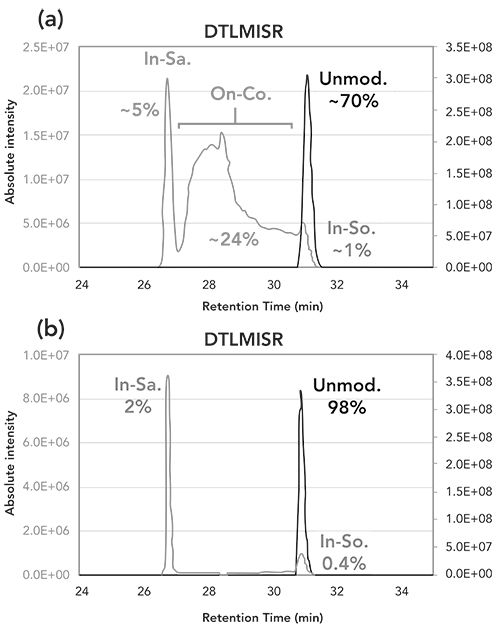
Figure 4: Orbital trap MS/MS spectra obtained by higher-energy collisional dissociation of the single protonated and (a) unmodified DTLMISR peptide, (b) in-sample oxidized, (c) on-column oxidized (28.0 min), (d) on-column oxidized (29.4 min), and (e) in-source oxidized DTLMISR peptides of the system suitability test sample using the aged column (see Figure 3a). Mass differences between the y4+ (m/z 506.28) and y5+ (m/z 619.36) fragment ions of the unmodified DTLMISR peptide, and the y4+ (m/z 522.27) and y5+ (m/z 635.35) fragments ions of the modified peptides demonstrate the methionine residues to be modified by +16 Da. In addition, the presence of neutral losses of 64 Da corresponding to methane sulfenic acid (CH3SOH) of the y4+ and y5+ fragment ions resulting in y4+-64 Da (m/z 458.27) and y5+-64 Da (m/z 571.36) further prove the modified peptides to be methionine oxidized.
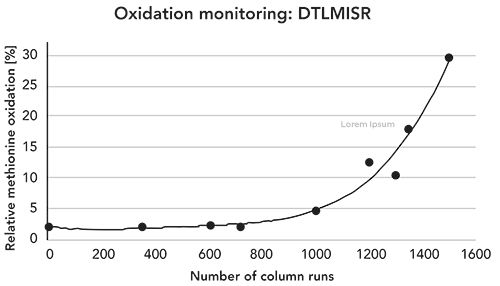
Following these observations, we investigated the correlation between the on-column methionine oxidation and the long-term column usage. By looking at the historical SST data generated on the aged column, the relative abundance of the methionine oxidized DTLMISR peptide was found to be dependent on the number of column runs (Figure 5). Until about 700–800 columns runs, the level of in-sample oxidation was constant at ~2% relative abundance. After ~1500 column runs, the oxidation of the DTLMISR peptide gradually increased to a total oxidation level of 30% (sum of in-sample, on-column, and in-source oxidation). Following these observations, monitoring of MetO has been included as part of the SST while quantifying protein oxidation by peptide mapping. This control minimizes the risk of oxidative artifacts and helps generate high quality data to provide reliable quantitative information on protein oxidation.
Figure 5: Relative abundance of methionine oxidized DTLMISR peptide (sum of in-sample, on-column, and in-source oxidation) of the SST sample dependent on the number of reversed-phase column runs as determined by extracted ion current chromatograms. With increasing on-column oxidation, the in-sample, on-column, and in-source oxidized peptides were not base-line separated.
On-column oxidation occurring on LC columns has been previously reported (24–25), and, in those studies, it was concluded that the oxidation reaction was due to metal catalysis of the column frits caused by trace levels of residual metal ions, most likely introduced from any surface in contact with the sample (25). A chelating agent ethylenediaminetetraacetic acid (EDTA) may be helpful to prevent or minimize the on-column oxidations and consequently extend the column lifetime. Also, the addition of antioxidants like methionine to the eluents could help protect the methionine containing peptides from oxidative damage.
Conclusions
In this study, we describe the impact of long- term column usage on the artificial increase of methionine oxidation during LC–MS/MS peptide mapping. Qualitative evidence for the on-column oxidation reaction was obtained by examination of the EIC chromatograms. The extent of the on-column oxidation significantly increased beyond 700–800 column runs, and the likely root cause is the presence of trace level metal ions on the chromatographic column. As a practical measure, we now visually/qualitatively monitor the level of on-column methionine oxidation of the SST sample tryptic peptide DTLMISR, which was oxidized to the highest level in this study.
Acknowledgments
The authors wish to thank the mass spectrometry team at Roche Innovation Center Munich for their support. We thank Cinzia Stella (Genentech) for carefully proofreading our manuscript.
References
- H.Z. Huang, A. Nichols, and D. Lu, Anal. Chem.81(4), 1686–1692 (2009).
- K. Diepold, K. Bomans, M. Wiedmann, B. Zimmermann, A. Petzold, T. Schlothauer, R. Mueller, B. Moritz, J.O. Stracke, M. Mølhøj, D. Reusch, and P. Bulau, PLoS One7(1), e30295 (2012).
- G. Xu and M.R. Chance, Anal. Chem. 77(14), 4549–4555 (2005).
- L. Fucci, C.N. Oliver, M.J. Coon, and E.R. Stadtman, Proc Natl Acad Sci USA80(6), 1521–1525 (1983).
- J.L. Liu, K.V. Lu, T. Eris, V. Katta, K.R. Westcott, L.O. Narhi, and H.S. Lu, Pharm Res.15(4), 632–640 (1998).
- E.R. Stadtman, Free Radic. Biol. Med.9(4), 315–325 (1990).
- E. Shacter, J.A. Williams, and R.L. Levine, Free Radic. Biol. Med. 18(4), 815–821 (1995).
- E. Shacter, Drug Metab. Rev.32(3-4), 307–326 (2000).
- S. Hermeling, D.J.A. Crommelin, H. Schellekens, and W. Jiskoot, Pharm Res.21(6), 897–903 (2004).
- D. Liu, D. Ren, H. Huang, J. Dankberg, R. Rosenfeld, M.J. Cocco, L. Li, D.N. Brems, and R.L. Remmele, Jr., Biochemistry47(18), 5088–5100 (2008)
- T.F. Lavine, J .Bio.l Chem.169(3), 477–491 (1947).
- W.E. Savige and A. Fontana, Methods Enzymol.47, 453–459 (1977)
- W. Vogt, Free Radic. Biol. Med.18(1), 93–105 (1995).
- A.V. Peskin and C.C. Winterbourn, Free Radic. Biol. Med. 30(5), 572–579 (2001).
- Y. W. Lao, M. Gungormusler-Yilmaz, S. Shuvo, T. Verbeke, V. Spicer, and O.V. Krokhin, J. Proteomics125, 131–139 (2015).
- B.L. Boys, M.C. Kuprowski, J. Noël, and L. Konermann, Anal. Chem.81(10), 4027–4034 (2009).
- R. D. Espy, M. Wleklinski, X. Yan, and R.G. Cooks, Trends Anal. Chem.57, 135–146 (2014).
- E.R. Stadtman, Annu. Rev. Biochem. 62, 797–821 (1993)
- J.O. Konz, J. King, and C.L. Cooney, Biotechnol. Prog.14(3), 393–409 (1998).
- J.C. Wahlich and G.P. Carr. J. Pharm. Biomed. Anal.8(8-12), 619–623 (1990).
- J.W. Dolan, LCGC Europe17(6), 328–332 (2004).
- W. Burkitt, P. Domann, and G. O’Connor, Protein Sci. 19(4), 826–835 (2010)
- J. Stracke, T. Emrich, P. Rueger, T. Schlothauer, L. Kling, A. Knaupp, H. Hertenberger, A. Wolfert, C. Spick, W. Lau, G. Drabner, U. Reiff, H. Koll, and A. Papadimitriou, MAbs6(5), 1229–1242 (2014)
- J.-X. Huang, J.B. Stuart, W.R. Melaner, and C. Horváth, J. Chromatogr. 316, 151–161 (1984).
- C. Tang, J. Tan, J. Jin, S. Xi, H. Li, Q. Xie, and X. Peng, Rapid Commun. Mass Spectrom.29(20), 1863–1873 (2015).
Björn Mautz, Vincent Larraillet, Maximiliane König, and Michael Mølhøj are with the Large Molecule Research group in Roche Pharma Research and Early Development (pRED) at the Roche Innovation Center Munich, in Penzberg, Germany. Direct correspondence to: michael.molhoj@roche.com

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.











