Method Development for Drug Impurity Profiling (Part 2)
LCGC Europe
A systematic approach to developing drug impurity profiling methods.
This article is the second part of the series on method development for drug impurity profiling. The first part described the first two steps: the selection of a set of dissimilar chromatographic columns (i.e., columns with different selectivities), and the selection of a suitable chromatographic column and optimization of the mobile phase pH. This second part will discuss the last two steps: optimization of the organic modifier composition and optimization of the gradient slope and the temperature.
An impurity is defined as 'any component of the new drug substance that is not the chemical entity defined as the new drug substance',1 and can be induced during drug synthesis, manufacturing and/or storage.1,2
To develop chromatographic impurity profiles by means of reversed-phase high-performance liquid chromatography (RPLC), different sequential steps can be considered, determined by the influence of the factors on the selectivity; (1) selection of a set of dissimilar chromatographic columns, (2) selection of a suitable chromatographic column from the above set and optimization of the mobile phase pH, (3) optimization of the organic modifier composition and (4) optimization of the gradient slope and the temperature.
In Part 1, the first two steps were discussed.3 After selection of a set of dissimilar chromatographic columns, the drug impurities mixture is screened on these four or five dissimilar columns at, for example, four different pH values, to select the most suitable column and the optimal pH value.
In Part 2, we will focus on the following two steps: (3) optimization of the organic modifier composition and (4) optimization of the gradient slope and the temperature. The best column and pH, selected in step (2), will be used to screen the mixture at different mobile phase compositions (different organic modifier compositions).
As already mentioned in the previous paper,3 it is not suitable to model either selectivity factors or resolutions (responses representing the separation degree or quality) directly as a function of the organic modifier fractions. Thus, for each compound (impurity) a model is built, relating its retention time (or factor) to the organic modifiers fractions. This allows predictions of the retention time (or factor) of each compound at intermediate organic modifiers compositions. Then, for each composition, the predicted retention times (or factors) and corresponding peak widths (considered constant in gradient elution and modeled for isocratic elution) are sorted from high to low, enabling the calculation of the selectivity factors or resolutions between consecutive peaks. The optimal organic modifiers composition is that where the minimal selectivity factor or the minimal resolution, such as the selectivity factor or the resolution of the worst separated peak pair, is the highest.
Optimization of the Organic Modifier Composition
The third step in developing drug impurity profiles consists of the optimization of the organic modifier composition. The chromatographic column and the mobile phase pH, selected from the second step, are used to screen the drug substance and its impurities at several isoeluotropic organic modifier compositions.
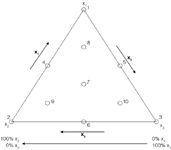
Figure 1: Solvents triangle, with x1, for instance, representing an ACN/H2O mixture, x2 MeOH/H2O, and x3 THF/H2O. Water content is determined by solvent strength. Numbers 1â10 (see Table 1).
In general, for a three-component mixture, for example with modifiers methanol (MeOH), acetonitrile (ACN) and tetrahydrofurane (THF), all possible compositions can be represented in a triangle (Figure 1). In Table 1 the compositions forming some mixture designs are shown. Each vertex of the triangle represents a condition where the organic part of the mobile phase only consists of one modifier. Each side represents binary mixtures of organic modifiers and inside the triangle ternary mixtures can be found. Additionally, each condition contains a given amount of water to make all compositions isoeluotropic. Any composition can be considered as a mixture with given fractions of x1, x2 and x3, each ranging between 0 and 1. When applying a gradient elution, isoleluotropy of the beginning and end conditions often is ignored. In fact in a gradient elution one moves from one triangle to another, from one with a larger water content to one with a smaller.

Table 1: Three-component mixture designs: (3,1) simplex lattice design with 3 (points 1 till 3) experiments; (3,2) simplex lattice design with 6 (points 1 till 6) experiments; (3,3) simplex lattice â centroid design with 7 (points 1 till 7) experiments; or augmented simplex lattice â centroid design with 10 (points 1 till 10) experiments.
A choice can be made between the optimization of either binary organic modifier mixtures alone, or binary and ternary organic modifier mixtures jointly. To limit the complexity of the mobile phase, one may initially chose to only use binary mixtures of organic modifiers when the vertex compositions do not result in baseline-separated chromatograms. In such situations, only one side of the triangle is explored (e.g., side 2 to 3 in Figure 1). Two different retention models can be constructed depending on whether the model is based on two or three measurements (Figure 2). The model for retention based on two measurements, [such as each vertex of one side of the triangle (points 2 and 3 in our example)], is a linear model (Equation 1). One based on three measurements, [such as each vertex of one side of the triangle (points 2 and 3) and a given point on that side of the triangle (e.g., point 6 in Figures 1 and 2)], is a quadratic polynomial model (Equation 2).
log(k) = b1x + b0 [1]
log(k) = b'11x2 + b'1x + b'0 [2]
where k is the retention factor, x the fraction of one of both modifiers (between 0 and 100% or between 0 and 1) and b1, b0, b'11, b'1 and b'0 are the regression coefficients of the models.

Figure 2: Optimization of a binary mixture of organic modifiers using (a) a linear model, and (b) a quadratic polynomial model to model retention. Si = substance i from mixture.
To optimize a binary mixture of organic modifiers, the drug mixture is analysed at two or three conditions and the model (Equations 1 or 2) is built for each impurity. In Figure 2, this is represented for four substances. For each intermediate mixture of organic modifiers, the retention is predicted for all impurities. At each composition, the retentions are then sorted and the selectivity factor α or the resolution Rs is calculated for each pair of consecutive peaks. To determine the optimal conditions, the minimal selectivity factor αmin or the minimal resolution Rsmin is plotted as a function of the composition of the mobile phase. The plot does not have a smooth behavior because the plotted αmin or Rsmin values at different conditions may originate from different peak pairs. That composition where αmin or Rsmin is maximal is then selected as the optimum, for example X = 40% x3 in Figure 2(a), meaning that 2/5 of composition x3 and 3/5 of composition x2 are mixed.
To explore the two other sides of the triangle, the above procedure is repeated for the sides 1 to 2 and 1 to 3. For the optimization of binary and/or ternary mixtures of organic modifiers (if you wanted to consider compositions in the entire triangle, for example), either three (points 1, 2 and 3 in Figure 1), six (points 1 to 6 in Figure 1), or seven (points 1 to 7 in Figure 1) experiments are executed.
The three-experiments (3,1) simplex lattice design (Figure 1 and Table 1, exp 1 to 3) is used to calculate the coefficients of the model.
log(k) or tR = b1x1 + b2x2 + b3x3 [3]
The six-experiments (3,2) simplex lattice design (Figure 1 and Table 1, exp 1 to 6) is used to calculate the coefficients of the model.
log(k) or tR = b1x1 + b2x2 + b3x3 + b12x1x2 + b13x1x3 + b23x2x3 [4]
The seven-experiments (3,3) simplex lattice–centroid mixture design (Figure 1 and Table 1, exp 1 to 7) is used to calculate the coefficients of the reduced cubic model.
log(k) or tR = b1x1 + b2x2 + b3x3 + b12x1x2 + b13x1x3 + b23x2x3 + b123x1x2x3 [5]
In Equations (3–5), k is the retention factor, tR the retention time, x1, x2 and x3 are the fractions of the first, second, and third organic modifier, respectively, and bi, bij and bijk are the model coefficients. Most applied when considering the entire triangle, is the latter situation based on seven experiments. Its model (Equation 5) is equivalent to the quadratic model we have been discussing earlier in the data handling column about response surface designs.5 Here, the model also represents a response surface.
The model coefficients of Equation 5 can simply be estimated from one or some measured results (Table 1).
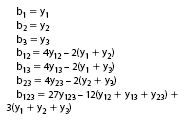
Experiments 8 to 10 (Figure 1 and Table 1) together with experiments 1 to 7 form another mixture design, for which the model of Equation 5 can be built. However, software is needed here to estimate the coefficients.
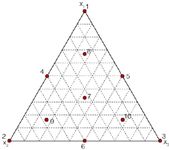
Figure 3: Visualization of the grid over the triangle.
To optimize a ternary mixture of organic modifiers, again a model is built for each impurity. Similar to the optimization of binary mixtures, for each intermediate composition of organic modifiers retention is predicted for all impurities. The intermediate compositions are determined by creating a grid over the triangle (Figure 3). At each composition, the retentions are then sorted and Rs or α is calculated for each pair of consecutive peaks. To determine the optimal conditions, αmin or Rsmin is plotted or visualized as a function of the composition of the mobile phase (Figure 4). That composition where αmin or Rsmin is maximal is then again selected as the optimum.
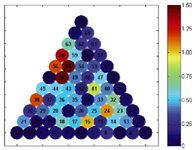
Figure 4: Visualization of the predicted minimal resolutions for the 66 grid points of Figure 3.
Optimization of the Gradient Slope and the Temperature
The fourth step in developing drug impurity profiles consists of the optimization of the gradient slope and the temperature. It concerns factors with less influence on the selectivity. This step often can be considered optional or rather as a fine-tuning of the method. It can be done using response surface designs. The types and properties of these designs were already discussed in reference 4. However, concerning the data analysis to determine the best conditions, again a similar approach, as in Part 1 for pH and here for organic modifier composition, is applied. Therefore, this approach will not be further elaborated here.
In general, the optimized conditions for two examined factors, x1 and x2 (e.g., gradient slope and temperature), to separate a mixture of compounds, is derived from a plot of Rsmin values at different x1–x2 grid conditions, as shown in Figure 5. Similar plots are obtained for the triangle (mixture domain) when plotting Rsmin values as a function of the organic modifier composition.
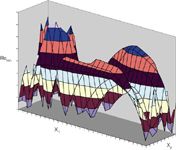
Figure 5: Plot of Rsmin values for the different grid conditions considered during the optimization of factors x1 and x2.
Conclusions
In this data handling column, step (3) optimization of the organic modifier composition and step (4) optimization of the gradient slope and the temperature, of the development of chromatographic impurity profiles were discussed in more detail.
Yvan Vander Heyden is a professor at the Vrije Universiteit Brussel, Belgium, department of Analytical Chemistry and Pharmaceutical Technology, and heads a research group on chemometrics and separation science.
Melanie Dumarey is a former PhD student from the same department [funded by a specialization grant awarded by the Institute for the Promotion of Innovation by Science and Technology in Flanders (IWT)], and is currently working as a postdoctoral fellow at the Umea University, Umea, Sweden.
Bieke Dejaegher is a postdoctoral fellow of the Fund for Scientific Research (FWO, Vlaanderen, Belgium) working at the same department on experimental designs and their applications in method development and validation, and on the development and data analysis of herbal fingerprints.
References
1. International Conference on Harmonisation of Technical Requirements for the Registration of Pharmaceuticals for Human Use (ICH) guideline Q3A(R2), Impurities in new drug substances, 2006, http://www.ich.org/, accessed on October 6th 2010.
2. S. Ahuja, Impurities evaluation of pharmaceuticals, Marcel Dekker, New York, 1998.
3. B. Dejaegher, M. Dumarey and Y. Vander Heyden, LCGC Europe, 23(4) 218–224 (2010).
4. B. Dejaegher and Y. Vander Heyden, LCGC Europe, 22(5) 256–261 (2009).
5. B. Dejaegher and Y. Vander Heyden, LCGC Europe, 22(11) 581–585 (2009).
Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.













