Meeting Review: Advances in Bio-Separations: Biologics Characterization from Cradle to Grave (Day Two)
The second part of the review on the two-day symposium focusing on the characterization of biopharmaceutical molecules at AstraZeneca’s MedImmune site in Cambridge, UK.
Paul Ferguson1 and W. John Lough2, 1The Chromatographic Society, Glasgow, UK, 2University of Sunderland, Sunderland, UK.
The second part of the review on the two-day symposium focusing on the characterization of biopharmaceutical molecules at AstraZeneca’s MedImmune site in Cambridge, UK.
Dr John Lough from the University of Sunderland, UK, chaired the session at the beginning of the second day. The session was a mixture of principally pharmaceutical presenters interspersed with presentations from instrument vendors highlighting their latest technologies relevant to the field. The first speaker in this session was Dr Milena Quaglia, LGC, who presented on “An experimental design protocol for protein structural analysis and the role of chromatography”. Dr Quaglia discussed why protein measurement was important in, for example, clinical markers, determination of food allergens, and in biopharmaceutical development. She then went on to discuss the complexities involved in the measurements of proteins, in particular their higher order structure because this provides information on the potency, efficacy, and safety of a bio-product. LGC are leading a programme to generate data to assist the selection of the most appropriate analytical platform for higher order protein structural analysis and to also benchmark novel platforms. One of these approaches involves the use of hydrogen-deuterium exchange, followed by trypsin digestion and peptide mapping with mass spectrometry (MS) detection. She discussed the effectiveness of this approach (which requires an understanding of deuterium back-exchange on the analytical column and optimization of digestion conditions) and compared the results with those generated using other techniques such as ion-mobility MS and capillary electrophoresis.
Figure 1: Symposium organizer Tony Taylor at the podium opening the meeting.

The second speaker in the morning session was Dr Nadim Akhtar from AstraZeneca (AZ) who presented on “Analytical Strategies for Oligonucleotides”. He further strengthened the contextual importance of the meeting topic by stating that biopharmaceuticals now make up 85% of all marketed drugs by sales value. Dr Akhtar has responsibility at AZ for the analysis of “new modalities” in the UK and it was instructive to hear how these species, which include oligonucleotides, fit into the AZ development portfolio. He opened his talk by discussing the limitation for small molecule therapeutics, in particular the inability of small molecules to effectively impact upon cellular functions, which are mediated by protein interactions with other proteins. He went on to discuss AstraZeneca’s interest in oligonucleotides, which are highly aqueous soluble short strands of DNA or RNA, the various forms these can take - the most common being siRNA (small interfering RNA) - and which are now being investigated for potential treatments of cancer, asthma, diabetes, and heart disease.
Figure 2: Symposium organizer Dr Chris Bevan with fellow former ChromSoc President, Alan Handley, at the Imperial War Museum Duxford.

Dr Akhtar listed the challenges associated with developing and filing oligonucelotides. ICH guidelines do not cover peptides and oligonucleotides. In fact they are specifically excluded. However, regulatory bodies expect these guidelines to be applied in spirit. There are also very few molecules that have reached the marketplace, so frequent regulatory interaction is key in delivering these immature (in terms of use as registered drugs) molecules. He discussed the impurity profiles generated using phosphoroamadite solid-phase chemistry and their impact on oligonucleotide purity. He also discussed other potential impurities, interestingly making the distinction “diastereomers rather than impurities” such as deletion sequences or modification of the sugar ring and the analytical techniques that are used to analyze for these impurities. Ion pairing reversed-phase LC is a key approach for analyzing these molecules based on their size and charge but chromatography is still extremely difficult and chromatograms are often “forced” into elution regions of shorter impurities, the main API and related impurities, and longer chain impurities. As chromatography lacks specificity to resolve the many possible isomeric species, MS is critical for mass-resolution, identification, and quantitation of impurities co-eluting with the API and requires very clean systems to avoid adduct formation. He completed his presentation by briefly discussing the complexity of the delivery systems required to target these drugs at the appropriate receptor, which will further add to the analytical challenge.
Following on from some vendor presentations, Dr Rob Wheller from GSK wound up the morning session with a presentation entitled “Platform for the Bioanalysis of Therapeutic Proteins Utilizing Immunocapture & Trapping LC–MS” in this session. He discussed the technology involved in the quantitative measurement of therapeutic proteins utilizing a magnetic bead processor for automated immunocapture, a trapping LC (utilizing an autosampler and UHPLC separation technology), and a mixer which can automate preparation steps for LC–MS workflows. He exemplified the approach through a case study with a monoclonal antibody and showed the impressive time-savings compared to the initial manual process (from 2.5 days down to 1.5 days). He concluded the presentation by discussing further potential improvements to the process including further automated sample preparation, flash digestion, and the use of an automated protein analysis workstation.
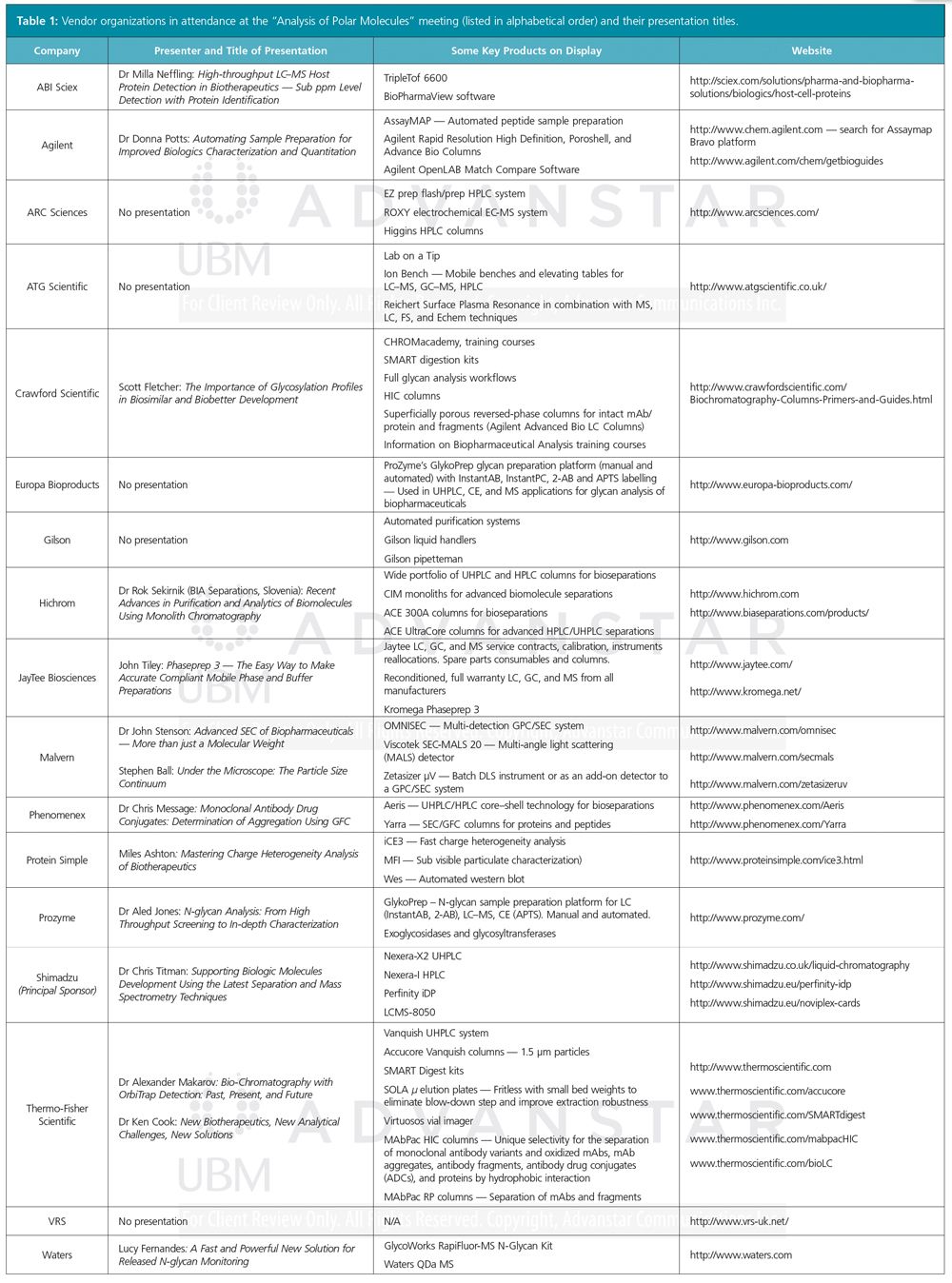
The final afternoon session included numerous vendor presentations highlighting a range of technologies and approaches for the analysis for all types of bioanalysis. The vendors’ products on display, titles of their presentations, and pertinent links to instrumentation or processes noted in their presentations are all summarized in Table 1. There were many highlights here but it would be unfair to exhibitors to single out particular examples as all companies provided a wealth of information.
So, it was left to Dr Chris Bevan of The Chromatographic Society to bring the meeting to a close by thanking the hosts, vendors, and delegates. Very encouraging feedback was received on the meeting from all attendees and undoubtedly the meeting topic should will be revisited in the future. The popularity of the meeting illustrated the interest in this area and the meeting helped highlight many of the potential solutions to the challenges of analyzing biopharmaceuticals. Nonetheless it was clear that the field of biopharmaceuticals is growing rapidly and will present separation scientists with enough interesting challenges to keep them busy for some time to come. Such meetings fulfil many requirements but a primary function is to allow delegates to get up to speed on all the key developments in a particular field quickly and effectively. This particular meeting was highly successful in fulfilling this primary function and The Chromatographic Society looks forward to playing its role in the development of chromatography by providing many such meetings in the future.
Paul Ferguson is a separation science specialist at a multinational pharmaceutical company in the UK working on both small- and medium-size novel therapies. He has worked in the pharmaceutical industry since 1999 following a post-doc at Imperial College London. Paul has particular interests in UHPLC, SFC, CE, chiral separations, formulated drug sample preparation, green analytical chemistry, and method development. He is a past winner of the Desty Memorial lecture prize (2002), and a Fellow and Chartered Chemist in the RSC. Paul is also the President of The Chromatographic Society in the UK and served as Vice-President for the Society from 2009 to 2014 where he was Chair of the Medals Committee conferring the Martin Gold and Jubilee medals. He has also organized or co-organized several successful symposia for the Society since he joined in 2007.
John Lough is a longstanding member of The Chromatographic Society Executive Committee, having fulfilled a variety of roles including President (2007–2009). At Sunderland, his pharmaceutical industry background, placement links, and research funding allowed him to play a leading role not only in the university’s BSc Chemical and Pharmaceutical Science course, which produced so many separation scientists for the pharmaceutical industry, but also in the later modernization of teaching provision, which led to the transformation into the current BSc BioPharmaceutical Sciences programme and the creation of the MSc Drug Discovery and Development programme, for which Dr Lough is Course Leader.
E-mail: chromsoc@meetingmakers.co.uk
Website: www.chromsoc.com

Determining the Link Between Prenatal Cannabis Use and Symptoms of Depression Using LC–MS/MS
April 16th 2025Researchers investigating the relationship between cannabis use during pregnancy and depressive symptoms—and whether continued use beyond the first trimester or higher levels of use were linked to increased symptoms—used liquid chromatography–tandem mass spectrometry (LC–MS/MS) to confirm the presence of 11-nor-9-carboxy-delta-9-tetrahydrocannabinol (THC-COOH) in urine samples.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.












