Characterizing Therapeutic Monoclonal Antibodies
Accounting for the inherent heterogeneity of monoclonal antibodies is essential to ensure production of consistent and safe therapeutics and, in light of this, there is an array of chromatographic and supporting regulatory systems. Robust characterization and analysis of monoclonal antibodies is becoming a fast and straightforward process thanks to the myriad technological advances underpinning this rapidly evolving area.
Chris Pohl, Thermo Fisher Scientific, California, USA.
Photo Credit: Travelif/Getty Images

Accounting for the inherent heterogeneity of monoclonal antibodies is essential to ensure production of consistent and safe therapeutics and, in light of this, there is an array of chromatographic and supporting regulatory systems. Robust characterization and analysis of monoclonal antibodies is becoming a fast and straightforward process thanks to the myriad technological advances underpinning this rapidly evolving area.
Ever since hybridoma technology proved to be a viable method of generating monoclonal antibodies (mAbs) in the 1970s,
1
the therapeutic application of immunoglobulins has exceeded all expectations and there are now hundreds of mAbs in clinical development. Currently, 39 novel mAbs are already in Phase 3 studies that are designed to target diseases including autoimmunity, inflammation, cancer, and infections.
2
At the current approval rate of novel therapeutics, we can expect approximately 70 marketed mAb products by 2020, along with $125 billion in combined global sales.
3
The success of mAbs as therapeutics stems from their selective and specific binding to proteins or molecules. However, unlike small molecule drugs, mAbs are produced from animals, cell lines, and fermentation processes, meaning they lack the purity of a chemically synthesized product. They can also be subject to possible degradation or chemical modification (“stresses”) during the manufacturing process that can affect biological activity and thus efficacy. Analytical and validation techniques, such as high performance liquid chromatography (HPLC), are essential for mAb quality control (QC) and characterization during drug development to ensure that they have safe, consistent, and stable properties that meet regulatory standards.
Chromatographic Characterization of mAbs
There are numerous chromatographic techniques used for the analysis and characterization of proteins, but the main techniques performed in mAb characterization are size-exclusion chromatography (SEC), ion exchange chromatography (IEC), and reversed-phase liquid chromatography (LC).
Size-Exclusion Chromatography (SEC):
SEC is the method most commonly used for characterizing the molecular weight distribution of samples and it is frequently used in the analysis of proteins to assess aggregate ratios and in protein purification. It works on the simple premise of separating molecules based on their molecular size, where the larger molecules elute first from the column. SEC columns can be polymer or silica-based, with the more recent columns employing a covalently modified diol hydrophilic layer that minimizes nonspecific binding of proteins to the underlying silica. Interactions between the sample and packing material should be avoided in SEC to have a true size-based separation.
Ion Exchange Chromatography (IEC):
IEC remains a very popular technique in the purification of therapeutic protein drugs. This technique utilizes the electrostatic interactions between the protein sample and the resin and can be neatly divided into either cation-exchange chromatography or anion-exchange chromatography. The choice between these two depends on the isoelectric point (pI) of the protein of interest and the pH at which the separation is performed. In salt-based elutions, the pH of the buffers used in the separation needs to be adjusted for every antibody according to its pI value. However, recent advances in the technology have made a dedicated pH gradient buffer system (covering a pH range of 5.6 to 10.2) available to perform the charge-based separation of antibodies on cation-exchange columns. This generic gradient technology is extremely useful in development environments where samples with a broad range of pIs are used and optimizing buffer systems for each sample would typically be a time consuming process. IEC has seen great success in monitoring modifications to proteins that change the overall charge.
4
Reversed-Phase Liquid Chromatography (LC):
Reversed-phase LC has very high resolving power and is often used to separate peptide mixtures that result from enzymatic digestion of a therapeutic protein. Separation of intact proteins can also be performed at a high resolution with LC, but requires dedicated columns. Mobile-phase temperature has also previously been shown to be an important factor for improving performance as well as limiting adsorption phenomena with mAbs.
5
In recent work
6
researchers performed LC with a column capable of thermostatting up to 120
o
C. Actively regulating the thermal balance between the mobile phase and the stationary phase limits the loss of efficiency because of temperature mismatch between the column and the incoming solvent. Four commercial antibodies (bevacizumab, cetuximab, rituximab, and trastuzumab) were eluted with a 10-min linear gradient. In all cases, the elution of the intact antibodies resulted in very sharp peaks.
6
Figure 1: Comparison between a reference (black trace) and a stressed antibody (blue trace). Gradient: 0–100% B in 10 min. (a) Full view and (b) enlarged view.
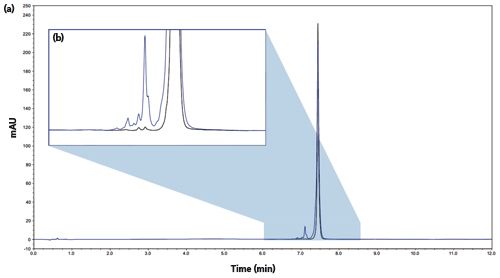
LC can also be used to assess the stability of an antibody in preliminary studies. To determine the effects of stress-related degradation on an antibody, researchers ran a simple 10-min gradient from 0 to 100% B (Figure 1) on a stressed and non-stressed sample.6 The increased relative area of the impurities eluting before the main peak of the stressed mAb confirmed the degradation of the sample. In addition, it is possible to estimate the degradation/denaturation of the sample by the increased width of the main peak. The width of the main peak at half height was 2.0 s for the reference, and 3.3 s for the stressed sample. Results such as these are likely caused by close-eluting species present in the stressed antibody but not in the reference one.
Supporting Techniques
To account for the inherent heterogeneity present not only within mAb production, but also the characterization steps, there needs to be regulatory guidance. The International Conference on Harmonization Guideline Q8 (R2) describes particular parameters of biopharmaceuticals, known as critical quality attributes (CQAs).
7
CQAs are defined as physical, chemical, biological, or microbiological properties or characteristics that should be within an appropriate limit, range, or distribution to ensure the desired product quality. Identifying CQAs appropriate to therapeutic mAbs is a challenging but necessary first step in the implementation of quality by design (QbD). Since mAbs are attributed with multiple important characteristics, such as purity, strength, potency, and safety, it is preferable to use multiple analytical techniques to identify these early on in the production process. Promotion of QbD through early assessment of CQAs and implementation of robust QC methods can help detect mAb heterogeneity, ensuring safety and efficacy, but also offering benefits to the industry in terms of guarding against unforeseen incidents and improving time to market. While HPLC and similar methods can be used to monitor CQAs throughout the development process, additional supporting techniques such as electrophoresis, spectroscopy, and sterility testing have essential roles to play. QbD is aligned with concepts from process analytical technology (PAT), and together these systems help to develop well-understood processes that will consistently ensure a predefined quality at the end of the manufacturing process.
8
Conclusion
Numerous techniques and supporting regulatory frameworks have been developed to promote consistency throughout mAb development and production. Each of the chromatographic techniques described here for the analysis and characterization of mAbs is associated with various advantages and limitations, meaning researchers need to be aware of changing trends and developments within the field to ensure that the selected method best suits their immediate and long-term goals. LC remains one of the most powerful and versatile techniques available for the characterization of mAbs and other molecules. The equipment and reagents used can now be tailored for a specific focus, using columns with smaller pore sizes for peptides, or including additives to the mobile phase for improved separation. With therapeutic mAbs looking set to continue their success in clinical settings, remaining on top of the latest chromatography developments is paramount for any researcher.
References
1. G. Köhler and C. Milstein,
Nature
256
(5517), 495–497 (1975). 2. J.M. Reichert,
MAbs
7
(1), 1–8 (2015). 3. D.M. Ecker, S.D. Jones, and H.L. Levine,
MAbs
7
(1), 9–14 (2015). 4. Thermo Scientific, http://www.thermoscientific.com/content/dam/tfs/ATG/CMD/cmd-documents/sci-res/app/chrom/lc/col/AN-21092-LC-CX-1-pH-Gradient-Buffer-Kit-mAb-AN21092-EN.pdf 5. S. Fekete and D. Guillarme,
LCGC Europe
25
(10), (2012). 6. Thermo Scientific, “Reversed-Phase Separation of Intact Therapeutic Antibodies Using the Vanquish Flex UHPLC System.” (2015). 7.http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q8_R1/Step4/Q8_R2_Guideline.pdf 8. A.S. Rathore, R. Bhambure, and V. Ghare,
Analytical and Bioanalytical Chemistry
398
(1), 137–154 (2010).
Chris Pohl
is Vice President, Chromatography Chemistry, at Thermo Fisher Scientific. He has more than 35 years of expertise in the design and development of chromatographic materials and holds 64 US patents along with numerous foreign equivalents.

Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Determining the Serum Proteomic Profile in Migraine Patients with LC–MS
April 17th 2025Researchers used liquid chromatography–mass spectrometry (LC–MS) in their proteomic analysis to compare the serum proteome of migraine patients with healthy controls and to identify differentially expressed proteins as potential migraine biomarkers.












