Measuring Water: The Expanding Role of Gas Chromatography
Special Issues
Daniel W. Armstrong

The accurate measurement of water content is an importunate and ubiquitous task in industrial and scientific laboratories worldwide. Water content is probably measured in a wider variety of matrices and at a greater range of concentrations (that is, less than parts per million to 99% +) than any other analyte. It must be measured in raw materials, final products, and a variety of natural systems. Often, there is a regulatory mandate to measure water in certain products including pharmaceuticals, foods, and other consumer products. Further complicating these measurements is the fact that water is an omnipresent impurity, which particularly complicates the accuracy and precision of any technique used to analyze samples for low levels of moisture.
Various analytical methods have been developed for the determination of water content. However, selection of the best method depends on the nature of the sample to be analyzed, the amount of water present, and the ultimate purpose of the information. Analytical approaches can differ completely for solid versus liquid versus gaseous samples or for trace versus moderate to high levels of water. A broadly useful technique that can be used for most sample types is highly desirable. Traditionally, the dominant approach for quantitating water has been Karl Fischer titration (KFT) despite its well-known limitations and inaccuracies (1). Recent developments in gas chromatography (GC) have elevated it to a position to challenge KFT. This change is largely the result of the development of new columns (based on ionic liquids) and detectors that are sensitive to water (1−5). Additionally, the ease of using capillary GC by either direct injection or in headspace formats greatly expands the types of samples that can be analyzed.
Ionic liquid stationary phases are known to have good thermal stability, but they are also completely stable in the presence of water and oxygen at high temperatures, so much so that humid air can be used as a carrier gas (see Figure 1). Obviously, a GC stationary phase that is used to separate water from other substances cannot be altered or degraded by water. Furthermore, the column must produce water peaks of good efficiency and symmetry so that area integration is accurate and reproducible. Finally, ionic liquid-based columns have optimal selectivity for separating water from a wide variety of polar and nonpolar substances (see Figure 2).
Figure 1: Comparison of repetitive injections of the same test mixture on a 30-m ionic liquid column (SLB-IL59, MilliporeSigma) and a conventional 30-m Carbowax column using air as the carrier gas. Thermal gradient from 50 °C to 200 °C in 30 min. Note the initial bleed and rapid decomposition of the Carbowax column, which does not occur with the ionic liquid column.
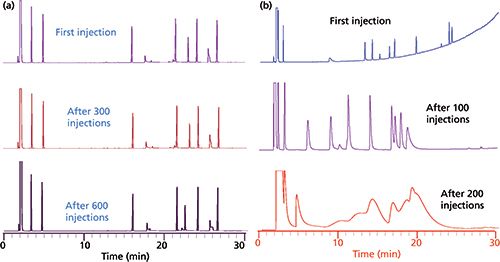
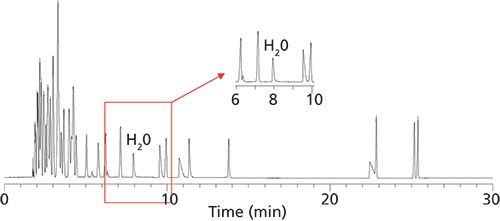
Figure 2: An example of how water is easily separated from 60 common organic solvents on a 30 30 m x 0.25 mm Watercol 1910 column (MilliporeSigma) and with good peak shape and efficiency. Carrier gas: helium; flow rate: 25 cm/s; temperature program: 45 °C, 5 °C/min to 75 °C (0 min), then 10 °C/min to 200 °C (2 min); sample injection volume: 1 µL; split ratio: 100:1; detection: thermal conductivity at 220 °C.
Three ionic liquid stationary phases were specifically developed for the separation and quantitation of water. Their structures are shown in Figure 3. These stationary phases have somewhat varied polarities and selectivities that provide optimal separations for different types of samples and matrices (vide infra). Equally important is the advent of new highly sensitive and “universal” GC detectors such as the barrier ionization discharge and vacuum ultraviolet detectors as well as advanced “ultradry” headspace devices (1−5). The determination of trace levels of water in any sample is dependent on the degree to which carrier and purge gases can be dried as well as the extent to which one can exclude atmospheric moisture from the entire analysis process. In fact, commercial gases (He, N2, He, Ar, and more) labeled as dry or anhydrous should not be used for ultratrace-level water determinations without further extensive purification (1). For example, the analysis of trace-level water in petroleum products (see Figures 4 and 5) require exceedingly dry conditions (1). Both the chromatographic quality and limit of detection (LOD) of the separation represented in Figure 4 could not be duplicated with a less sensitive detector (for water) or with moisture contamination of the flow gas or the rest of the system.
Figure 3: Structures of the three polar ionic liquid stationary phases that were specifically designed and synthesized by the Armstrong research group for the GC analysis of water (1−
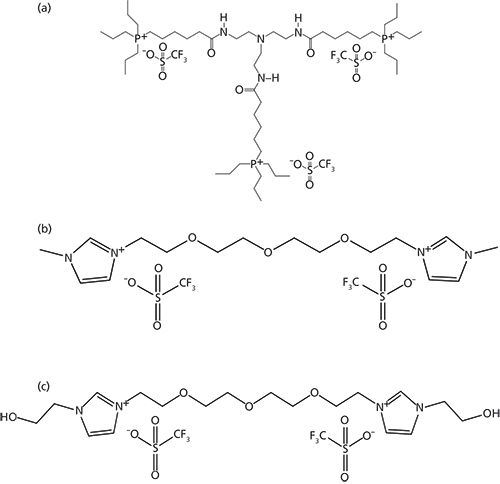
6).
Figure 4: Direct injection determination of 25 ppm water in a liquid petroleum gas sample using a 30-m Watercol 1910 column and a Shimadzu Tracera GC-2010 + BID-2010 Plus barrier ionization discharge detector. Injection volume: 2 µL; split ratio: 1:5; temperature program: 35 °C for 2.0 min, then 35−
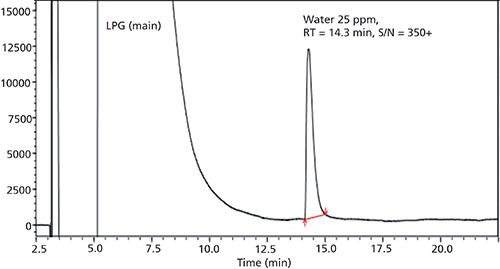
150 °C at 5 °C/min (hold at150 °C for 15 min), total = 40 min; carrier gas: helium; linear velocity: 45 cm/s. Note that the limit of detection (LOD) and limit of quantitation (LOQ) for water were determined to be 0.22 and 0.66 ppm, respectively. Adapted with permission from the Shimadzu App. Data Sheet No. 18, 2017.
Figure 5: Chromatogram of unleaded gasoline spiked with water. Separation done with a 30-m Watercol 1910 column with thermal conductivity detection. Carrier gas: helium; flow rate: 20 cm/s; temperature gradient: 45 °C (4 min), 5 °C/min to 75 °C, then 10 °C/min to 220 °C (10 min).
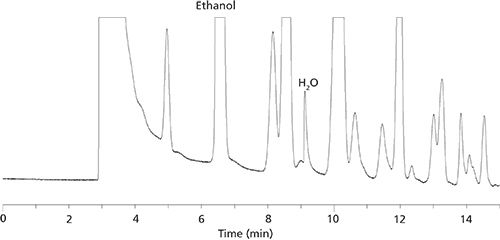
The preferred approach for solid samples is headspace GC. The solid samples are dissolved in a headspace solvent in a purged-sealed vial. The vial and sample are heated to enhance the moisture content in the headspace, which is sampled and injected into the GC. For such analyses, it has been found that dry ionic liquids make the best headspace solvents since they are nonvolatile and do not show up in the chromatogram as do all other headspace solvents (1,2,5). This approach has been shown to be effective for pharmaceutical products and their active pharmaceutical ingredients (APIs) (2,5). Headspace GC also is an effective approach for quantitating water in many types of food products (4,6). Also, both water and ethanol are easily measured in most alcoholic beverages and all other solvents using either direct injection or headspace GC (3,7).
The new instrumentation specifically designed for GC water analysis (for example, columns, detectors, headspace samplers, and flow gas purifiers) make GC the first truly competitive and often superior approach to KFT. Its speed, accuracy, and ease of automation offer advantages that are difficult to match with KFT or other techniques. Finally, very little sample is needed for direct GC or headspace GC (from about 0.50 to 500 µL, respectively). It appears that with the recent developments in GC and headspace GC, they are well positioned to make a significant impact in the way in which water is measured.
Acknowledgment
Structures and some material for figures were supplied by Leonard M. Sidisky, Millipore Sigma.
References
- L.A. Frink and D.W. Armstrong, Anal. Chem.88, 8194–8201 (2016).
- L.A. Frink, C.A. Weatherly, and D.W. Armstrong, J. Pharma. Biomed. Anal.94, 111–117 (2014).
- C.A. Weatherly, R.M. Woods, and D.W. Armstrong, J. Agricultural and Food Chem.62, 1832–1838 (2014).
- L.A. Frink and D.W. Armstrong, Food Chem.205, 23–27 (2016).
- L.A. Frink and D.W. Armstrong, J. Pharm. Sci.105, 2288–2292 (2016).
- L.A. Frink and D.W. Armstrong, LCGC North Am., “Advances in Food and Beverage Analysis” supplement 34(s10), 6–13 (2016).
- D.A. Jayawardhana, R.M. Woods, Y. Zhang, C. Wang, and D.W. Armstrong, LCGC Europe24, 516–529 (2011).
Daniel W. Armstrong is the Robert A. Welch Distinguished Professor in the Department of Chemistry and Biochemistry at The University of Texas at Arlington in Arlington, Texas.

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.




















