Redefining Simplicity in Ionization: Discovery and Implementation of Novel Ionization Processes in Mass Spectrometry
Special Issues
Sarah Trimpin

Mass spectrometry (MS) is a powerful tool for characterizing exceedingly low amounts of compounds in complex materials by separating gaseous ions according to their mass-to-charge ratio (m/z) with exceedingly high mass resolving power (>106 m/Δm, 50% peak overlap at low m/z) for some instruments. A severe MS limitation, for a number of years, was the inability to convert nonvolatile compounds to the gas-phase ions. Electrospray ionization (ESI) and matrix-assisted laser desorption-ionization (MALDI) are the most prominent ionization methods to open MS to the analysis of nonvolatile and high-mass compounds, including proteins. Specialized mass spectrometers and ion sources were built, beginning in the late 1980s, to accommodate these powerful ionization methods despite disagreements over ionization mechanisms and the knowledge needed to enhance them. For almost 30 years, considerable effort has gone into improving instrumentation relative to these ionization methods.
More recently, ionization processes were discovered and developed into analytical ionization methods that produce ions from various materials by solely having either a small-molecule solid or solvent matrix present when exposed to the subatmospheric pressure at the mass spectrometer inlet (1). The simplest of these methods, matrix-assisted ionization (MAI), spontaneously transfers compounds into gas-phase ions without the need for voltage, photons, or even added heat (2). While the fundamentals are not yet well understood, these simple processes have excellent sensitivity with low attomole limits of detection (3) and produce clean full-scan mass spectra requiring only a few femtomoles of samples such as drugs and peptides. In spontaneous ionization any means of introducing the matrix-analyte sample into the sub-atmospheric pressure of the mass spectrometer produces analyte ions.
MAI is part of a family of inlet and vacuum ionization technologies that achieve charge separation primarily through temperature and pressure differential with or without the aid of collisions. In these ionization processes, the matrix is key, and it can be a solid or a solvent that is introduced to the subatmospheric pressure with (for example, tapping) or without (that is, by direct insertion) the use of a force. In any case, the use of a laser, high voltage, and nebulizing gas is not necessary and clogged capillaries are eliminated--all items that increase cost of analysis and subject the analysis to failure. Gas-phase ions are observed multiply-protonated or deprotonated, depending on the polarity selected by the mass spectrometer, just like in ESI, even using aprotic matrices such as 3-nitrobenzonitrile (3-NBN) (4,5). MAI matrices that require no heat sublime in vacuum, and thus are pumped from the mass spectrometer ion optics, similar to solvents used with ESI. Heating the ion transfer tube of the mass spectrometer increases the number of matrices capable of producing high sensitivity even from solutions; this technique is called solvent-assisted ionization (SAI) (6). SAI and a related technique, voltage-SAI, were used with liquid chromatography (LC) to achieve higher sensitivity than LC–ESI-MS without the need for nebulizing gases or special emitters (7,8).
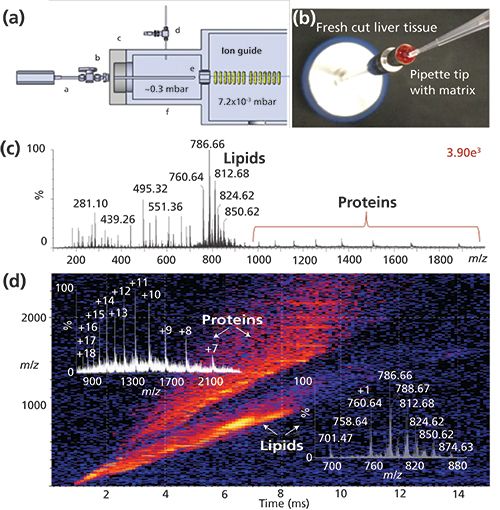
The initial developments of these technologies were accomplished using commercial ion sources of different vendors’ mass spectrometers. However, most commercial sources are not equipped for optimum MAI. Efforts designing and building dedicated inlets promise a better understanding, performance, and universal vendor applicability of the new ionization processes (9). Consequently, in collaborative efforts, dedicated sources are being developed that allow complex samples to be directly analyzed using a probe-type vacuum introduction to any mass spectrometer (10), and tube inlets for sample introduction directly from atmospheric pressure with and without LC (11), manually and automated. For example, complex mixtures can now be acquired in seconds directly from biological fluids, tissue, or buffered solution without any potential cross-contamination (Figure 1), or from surfaces such as dollar bills using a vacuum cleaner approach in a contactless fashion. By placing matrix only on the feature of interest on a surface (Figure 1b) and exposing the surface to the vacuum of the mass spectrometer, ions are observed from compounds exposed to the matrix solution allowing direct, rapid interrogation of “features of interest.” With direct analysis of biological materials, the extreme complexity can be overwhelming (Figure 1c), and additional separation, other than m/z, is desirable. Ion mobility spectrometry (IMS) interfaced to MS adds another nearly instantaneous gas-phase separation (Figure 1d) cleanly separating proteins from the lipid ions contained in the tissue and providing distinctive drift-time distributions for each ion, similar to the retention time in LC–ESI-MS. IMS also increases the dynamic range of an experiment; however, it does not address the issue of ion suppression in mixtures. Ion suppression is best reduced by a separation process before ionization, but solvents used in LC also contribute to ion suppression effects in ESI. Other reasons for using LC before MS include improved quantitation, especially when isotopic internal standards are not available. Combining LC or IMS with high-resolution MS and MS/MS provides exceptionally powerful approaches for analysis of complex mixtures (12). However, there are numerous instances where the added difficulty, time, and cost can be eliminated by using direct ionization methods as witnessed by the growth in so-called ambient ionization (13).
Figure 1: A dedicated vacuum MAI source using a probe on a Waters Synapt G2S IMS-MS mass spectrometer: (a) designed and constructed to analyze samples directly (9). (b) Bovine liver was placed on the probe and MAI matrix 3-NBN solution added onto the tissue, briefly dried, after which the probe is inserted into the vacuum housing through a ball valve. Only volatile material expands into the gas phase of the mass spectrometer avoiding source contamination. (c) Total mass spectrum. (d) IMS-MS detection readily visualizes various chemical components of the tissue analyzed. Insets display the mass spectra and charge state assignments for lipids and proteins extracted from the 2D dataset, respectively. Legend in (a): a = probe, b = vacuum lock, c = flange, d = gas control valve, e = commercial inlet cap, and f = housing.
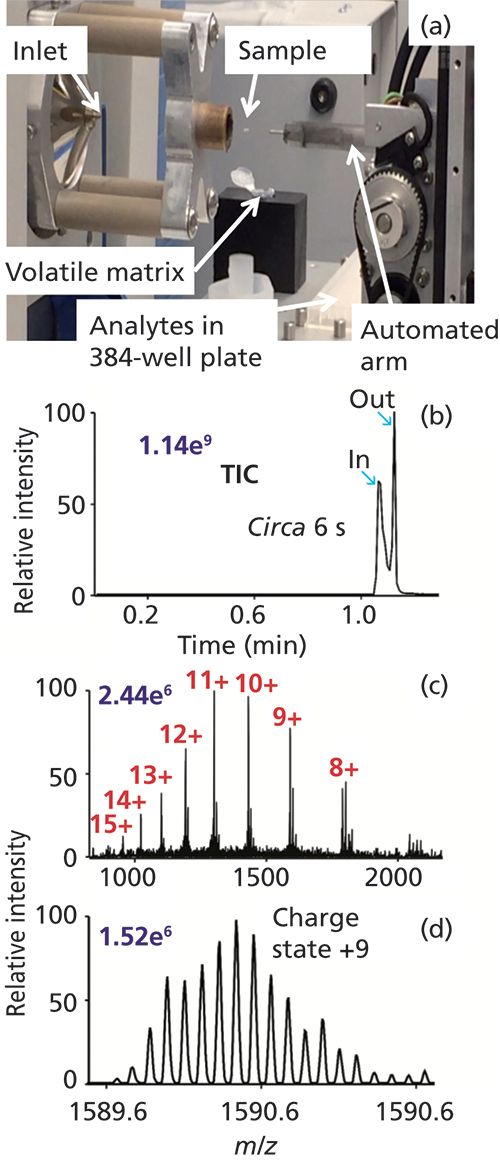
Inlet ionization methods are well suited either for single- or multiple-sample analyses. One of the first such demonstrations was a multiplexed, contactless SAI method rapidly characterizing sample composition from well-plates using imaging software (14). Taking full advantage of simple, flexible, rapid, and robust ionization technology, an automated prototype platform was developed capable of MAI, SAI, vSAI, and ESI from 384-well plates. For example, automated MAI-MS within approximately 6 s analyzed lysozyme, 14.3 kDa, from a single well at 140,000 mass resolution (Figure 2).
Figure 2: MAI using 0.1 µL of a 5 pmol/µL lysozyme solution combined with 3-NBN matrix promptly crystallized on the fused-silica tip, within ca. 6 s of data acquisition at 140,000 mass resolution. (a) Photograph of automated MAI system, (b) total ion chronogram, and mass spectra (c) total and (d) inset. Other source modules include SAI, vSAI, and ESI. Matrix 3-NBN (150 mg) was dissolved in 4 mL of acetonitrile-water in a ratio of 3:1 (v/v). A movie can be accessed at http://mstmllc.com.
As with any unique new ionization process, the range of applicability will expand--as has already been demonstrated with a number of samples obtained from diverse ionization platforms where ESI and MALDI either failed or produced poor results. These favorable results are based primarily on improved selectivity for the compounds of interest rather than on more-universal ionization. Further expansion of matrices and solvents will likely provide better selectivity and more-universal ionization capabilities. For a wide range of compound classes, such as drugs and their metabolites, lipids, peptides, and proteins, the simple and robust ionization methods provide improved data quality with competitive and frequently improved sensitivity relative to ESI and MALDI. The new ionization processes have shown unique applications with instruments ranging from small portable systems to ultrahigh-resolution mass spectrometers, and have been used with LC, IMS, and advanced fragmentation technology such as electron transfer dissociation. Areas that will likely benefit the most from these low-cost ionization processes are those that are in dire need of simplicity, robustness, throughput, and field portability, such as biothreat detection, biomedical research, and eventually point-of-care diagnostics.
Acknowledgment
Any opinions, ï¬ndings, and conclusions or recommendations expressed in this material are those of the author and do not necessarily reï¬ect the views of the National Science Foundation. NSF CHE-1411376 and STTR Phase II 1556043 are kindly acknowledged.
References
- S. Trimpin, J. Am. Soc. Mass Spectrom. 27, 4–21 (2016).
- E.D. Inutan and S. Trimpin, Mol. Cell. Proteomics 12, 792–796 (2013).
- K. Hoang, M. Pophristic, A.J. Horan, M.V. Johnston, and C.N. McEwen, J. Am. Soc. Mass Spectrom.27, 1590–1596 (2016).
- S. Trimpin and E.D. Inutan, J. Am. Soc. Mass Spectrom. 24, 722–732 (2013).
- S. Trimpin, C.A. Lutomski, T.J. El-Baba, D.W. Woodall, C.D. Foley, C.D. Manly, B. Wang, C.W. Liu, B.M. Harless, R. Kumar, L.F. Imperial, and E.D. Inutan, Int. J. Mass Spectrom.377(SI), 532–545 (2015)
- V.S. Pagnotti, N.D. Chubatyi, and C.N. McEwen, Anal. Chem.83, 3981–3985 (2011).
- V.S. Pagnotti, N.D. Chubatyi, A.F. Harron, and C.N. McEwen, Anal. Chem.83, 6828–6832 (2012).
- M.A. Fenner, S. Chakrabarty, B. Wang, V.S. Pagnotti, K. Hoang, S. Trimpin, and C.N. McEwen, Anal. Chem.89, 4798–4802 (2017).
- S. Trimpin, B. Wang, E.D. Inutan, J. Li, C.B. Lietz, V.S. Pagnotti, A.F. Harron, D. Sardelis, and C.N. McEwen, J. Am. Soc. Mass Spectrom.23, 1644–1660 (2012).
- I.C. Lu, M. Pophristic, E.D. Inutan, R.G. McKay, C.N. McEwen, and S. Trimpin, Rapid Commun. Mass Spectrom.30, 2568–2572 (2016).
- I.C. Lu, E.A. Elia, W.J. Zhang, M. Pophristic, C.N. McEwen, and S. Trimpin, Anal. Methods DOI: 10.1039/C7AY00995J (2017).
- X. Liu, S.J. Valentine, M.D. Plasencia, S. Trimpin, S. Naylor, and D.E. Clemmer, J. Am. Soc. Mass Spectrom.18, 1249–1264 (2007).
- P.M. Peacock, W.J. Zhang, and S. Trimpin, Anal. Chem.89, 372−388 (2017).
- B. Wang and S. Trimpin, Anal. Chem.86, 1000–1006 (2014).
Sarah Trimpin is Professor of Chemistry at Wayne State University in Detroit, Michigan, and the co-founder and CEO of MSTM, LLC, in Newark, Delaware.

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).




















