Looking for Carcinogenic Nitrosamine Compounds in Beer
Carcinogen-containing compounds can develop through some manufacturing processes, such as in the malting process for beer. It is critical to analyze the nitrosamine content in any consumed products as well as monitor the final product. Thermal energy analysis (TEA) is able to quickly identify and analyze N-Nitro, N-nitroso, and nitrogen-containing compounds. This article describes the importance of monitoring these compounds.
Photo Credit: Love the wind/stock.adobe.com

Carcinogen-containing compounds can develop through some manufacturing processes, such as in the malting process for beer. It is critical to analyze the nitrosamine content in any consumed products as well as monitor the final product. Thermal energy analysis (TEA) is able to quickly identify and analyze N-Nitro, N-nitroso, and nitrogen-containing compounds. This article describes the importance of monitoring these compounds.
A common blood pressure control drug was recently recalled in many countries because it contains a chemical that poses a potential cancer risk (1).
The US Food and Drug Administration recall later expanded to other drugs that contained valsartan (2). Valsartan is used as a component in a family of drugs used to treat heart failure and high blood pressure. During tests of valsartan’s active pharmaceutical ingredient (API), those from an external supplier contained an impurity, namely N-Nitrosodimethylamine (NDMA). NDMA is an organic chemical that is in a family of potent carcinogens (3).
NDMA has previously been used to make liquid rocket fuel, softeners, and lubricants, among other products. NDMA can also be unintentionally produced through certain chemical reactions, and can be a byproduct from the manufacture of pesticides, the making of rubber tyres, or fish processing (4). Animal studies have shown that NDMA can be toxic and cause tumours in the liver, kidney, and respiratory tract. It can also be potentially harmful to humans in certain quantities. Exposure to high levels can cause liver damage and is a probable human carcinogen, according to the US Department of Health and Human Services (5).
The same compound is detected and analyzed across other markets, including in the brewing industry. NDMA was discovered in beer in the 1970s. The Maltster’s Association of Great Britain (MAGB) members have implemented a voluntary code to monitor and report on levels in malt, as well as developing best practices to reduce the formation of the compounds during kilning. Malts are regularly tested by malting companies for NDMA formation, and the MAGB collates their members’ thousands of test results over the years to demonstrate due diligence action (6).
Detecting NDMA in Malt and Beer
During the malting process, barley is forced to germinate, after which the grain is dried in kilns. This process freezes the sugar and flavour compounds for the brewing process. During the kiln drying process, nitrosamines may be formed in the grain, which could remain within the extract and still be present in the final brewed product. Techniques were recently developed to minimize the formation of nitrosamines. However, low levels of these carcinogenic compounds still remain. Therefore, all malt used in the brewing process needs to be analyzed for its nitrosamine content. It is important to monitor the final product as well as the malt, to help regulate the exposure of nitrosamines from the consumed liquid.
Application Example
Method: A 200 Series Gas Chromatograph (Ellutia), with an 800 series TEA (Ellutia) was used for the analysis The conditions for testing and sample preparation are described here.
GC Conditions: Injector temperature: 250 °C; liner type: focus liner with wool; carrier gas type: helium; carrier gas control method: constant flow; splitless time: 0.8 min; column flow: 1.0 mL/ min; injection volume: 1 μL; column: 30 m × 0.25 mm, 0.25-μm EL WAX (Ellutia).
Column Temperature Program: Initial temperature: 45 °C/hold 1 min; temperature ramp 1: 20 °C min; column temperature 1: 130 °C/0 min hold time; temperature ramp 2: 200 °C (10 °C/min); column temperature: 230 °C/1 min hold time.
TEA Conditions: Pyrolyzer temp: 500 °C; interface temp: 250 °C; sensitivity: 250; pump type: Edwards nXDS10i.
Sample Preparation: Malt samples were extracted in duplicate. Each replicate had 50 g of malt ground up and 100-mL deionized water added. The extract was filtered through a grade 1 filter paper and 1 mL of 10 ppm NDPA (n-nitroso di propylamine) internal standard was added to one extract (this generates a 100 ppb NDPA spiked sample). The samples were then made up to 100 mL with deionized water volumetrically.
To a vial, 10 mL of extract, 3 g of sodium chloride, and 10 mL of dichloromethane (DCM) were added and shaken for 5 min. The layers were then left to separate for 15 min. The lower layer containing DCM was pipetted out into a clean vessel. A 10-mL measure of DCM was added to the extract and the liquid–liquid extraction step was repeated. After this step, the DCM (final volume approximately 20 mL) was dried using 1 g of sodium sulphate and then preâconcentrated to 1 mL under a nitrogen flow of approximately 1 L/min. A 1-μL injection of the concentrated DCM was directly analyzed.
The identity of the internal standard was confirmed against the eight-component nitrosamine mix standard. This confirmed that the NDPA internal standard used has the same retention time as the NDPA contained within the standard (Figure 1).
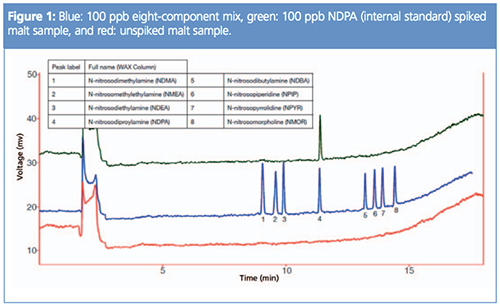
The peak areas for the spiked sample compared with the standard for NDPA showed good correlation, indicating a good recovery of the internal standard, and indicated very limited losses of any potential nitrosamines from within the sample during the preparation steps. The unspiked sample showed no peaks within the retention times of any of the nitrosamines in this standard mix. As such, this malt sample showed no observable response for NDMA or any other nitrosamines.
Beer
A 25-mL measure of lager was sonicated for 10 min to remove any dissolved gases. Ten mL was transferred to a centrifugal tube, 3 g of sodium chloride was added, followed by 1 mL of DCM, and the solution was shaken for 5 min. The sample was then left to separate out for 15 min. The DCM layer was pipetted into a new vial and 1 g of sodium sulphate was added to capture any remaining aqueous solution. A 1-μL injection of the DCM solution was directly analyzed.
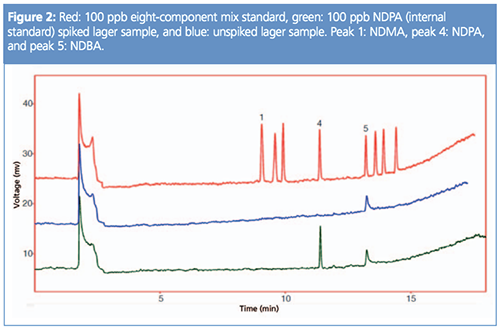
NDMA detection limits show that <1 ppb NDMA was visible. For the malt sample, the results would be declared as none detected <1 ppb, the beer would also be declared as <1 ppb NDMA, however, there was a response found for NDBA (n-nitrosobutylamine) of 67 ppb, highlighting the importance of looking for more nitrosamines than just NDMA (see Figure 2).
Conclusions
Following the US FDA’s alert for the voluntary recall of several drug products containing the active ingredient valsartan during the summer of 2018, the NDMA impurity was brought to light and found to have been in the recalled products. This article has described the similarities that occur in the brewing industry with the same impurity, when, during the kilning of germinated barley, in the course of a malting operation, nitrosamines are formed and, ultimately, end up in the beer, whisky, and vinegar obtained from such malt.
There are, however, measures in place to test for this in the brewing industry, but not in the pharmaceutical industry. The recent valsartan issue shows that nitrosamine testing should be part of certain pharma products safety tests. Therefore, it is important to test for this impurity and install this as an automatic part of the process.
References
- Jen Christensen, 2018. CNN - Common heart drug recalled in 22 countries for possible cancer link: https://edition.cnn.com/2018/07/06/health/valsartan-heart-drug-recall-intl/index.html
- Jen Christensen, 2018. CNN - FDA expands recall of blood pressure drug valsartan due to cancer concern: https://edition.cnn.com/2018/08/08/health/valsartan-recall-fda-expanded/index.html
- United States Environmental Protection Agency (EPA), 2014. Technical Fact Sheet –N-Nitroso-dimethylamine (NDMA) https://www.epa.gov/sites/production/files/2014-03/documents/ffrrofactsheet_contaminant_ndma_january2014_final.pdf
- Jen Christensen, 2018. CNN - FDA joins 22 countries’ recall of common heart drug. (Internet) https://edition.cnn.com/2018/07/13/health/valsartan-recall-fda-bn/index.html
- Environmental Protection Agency, 2017 - Technical Fact Sheet –N-Nitroso-dimethylamine (NDMA) November. https://www.epa.gov/sites/production/files/2017-10/documents/ndma_fact_sheet_update_9-15-17_508.pdf
- Food Safety in Malting and ‘Due Diligence’. The Maltster’s Association of Great Britain (MAGB). (Internet) http://www.ukmalt.com/food-and-feed-safety#Food%20safety
Andrew James is Marketing Director at Ellutia. Andrew has worked at Ellutia for over 20 years and has been involved with many aspects of the business from product development to strategic planning. Consequently, he has developed an extensive wealth of knowledge and experience in the chromatography industry. Andrew has been in charge of the company’s marketing for the last eight years, working to continually grow both the Ellutia brand and company as a whole.
E-mail:Andrew.James@ellutia.comWebsite:www.ellutia.com

Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.