A Look Back and A Look Forward—An Annual Check‑up on the State of Sample Preparation
LCGC Europe
As this is the final “Sample Prep Perspectives” column of the year, it is fitting to assess the state of the field by taking a look back and a look forward.
As this is the final “Sample Prep Perspectives” column of the year, it is fitting to assess the state of the field by taking a look back and a look forward. Specifically, we’ll start by addressing a reader’s email, then look at the state of sample preparation at two recent conferences. In the August issue, we looked at the role of blanks (samples lacking the analyte of interest used to determine or track the source of contamination or sample degradation and taken through the analytical process) in understanding the sample analysis process. One reader suggested that it is appropriate to address the Total Youden Blank, an often overlooked and perhaps the most true blank. The Total Youden Blank completely eliminates the constant error component arising from any source of bias involved in the measurement. In August 2017, we lost Dr. Patrick McDonald. McDonald was a Research Fellow at Waters Corporation and one of the pioneers in the development of solid-phase extraction (SPE). At the Fall National Meeting of the American Chemical Society, a symposium was held in his memory. We’ll review the symposium and McDonald’s contributions to get an up-to-date snapshot of the field of SPE. Finally, at this summer’s ExTech (International Symposium on Extraction Technologies), an expert panel offered their views on the future of sample preparation. A summary of this panel discussion is presented.
Since I took responsibility for this column, I’ve tried to focus on techniques, with a balance of theory, applications, and operational aspects. I’ve aimed to provide a balance that will be of interest to both the bench chemist and the laboratory supervisor, at all levels of education and background. Occasionally, trends related to sample preparation are reported. This month, the final instalment of the year will take a slightly different approach. Three vignettes are presented, either looking back at important, but overlooked, developments, or prognosticating the future.
The Total Youden Blank
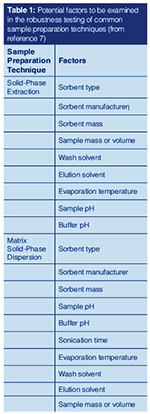
In our previous column (1), we discussed the role of sample blanks in chemical analysis, and provided an overview of various types of blanks. One concerned reader sent an e-mail to comment on an often overlooked, but highly important, type of blank, the total Youden blank. From the mid-1940s to the early 1970s, W.J. Youden presented research on the application of multivariate analysis to data sets from chemical determinations (2–6). The calibrations and methods presented determine, among other uses, constant errors with proposed calculations to confront proportional errors. These methods use a fractional factorial design to minimize the number of analyses in assaying several factors. In single laboratory validations, the susceptibility of analytical methods to small changes in parameters is examined. For example, in sample preparation, we have several optimization parameters of varying importance, as illustrated in Table 1 (7). This list of optimization parameters is not exhaustive, but limited to the major influences. Other extraction methods, and each step in an analytical procedure, will contribute different, and additional, optimization parameters. Evaluating each of these in a one-atâaâtime approach would be prohibitive. When analytical signals are due solely to the presence of analyte, with no matrix-based interference, the signal from the analyte [SA] can be modelled as [SA] = K + [αA]CA, where K is the Youden blank or “true sample blank,” [αA] is the slope of the calibration curve (or analytical sensitivity), and CA is the analyte amount (either concentration or weight). Several reviews or tutorials, such as those in references 7–10, provide more detailed treatments of the Youden method.
In Memory of Patrick D. McDonald and Recent Advances in Solid-Phase Extraction Symposium

One of the pioneers in the development of solid-phase extraction (SPE), Patrick D. McDonald, passed in August 2017. Dr. McDonald (Figure 1) was hired by Waters and Associates in 1974, initially to develop preparative liquid chromatography (LC) instruments. Later, he was charged to “find new, faster, more convenient ways to perform traditional sample preparation operations (11).” While others have used adsorbents in sample cleanâup procedures, his team went from proposing the use of LC technology in a June 1977 internal memo to shipping the first “Sample Enrichment and Purification” (SEP-PAK) product in January 1978. SEP-PAK featured a heat-shrinkable polyethylene body, triaxial compression technology, and preparative LC C18-silica particles. Figure 2 shows the cover of the original February 1978 marketing brochure for SEP-PAK cartridges. Note that these questions concerning the cost, time, and interferences of sample preparation still resonate today, though it is hoped that sample preparation advances have significantly improved over the decades and the current situation reflects the simultaneous advances in analysis techniques! As SEPâPAK cartridges became accepted and other manufacturers developed other approaches, J.T. Baker’s term, solidâphase extraction (SPE), came to represent the technique.
McDonald also edited Waters’ “SolidâPhase Extraction Applications Guide and Bibliography.” By the sixth edition in 1995, over 3000 applications were compiled. In 1996, he and his team developed and patented the Water’s Oasis HLB copolymer (12). This sorbent is a hydrophilic-lipophilic balance copolymer. The water-wettable sorbent retains analytes over a wide polarity range and is stable from pH 1–14.

In memory of McDonald, a symposium on recent advances in SPE was held at the 256th National Meeting of the American Chemistry Society at Boston, USA, in August, organized by Tom Walter, a Corporate Fellow at Waters and former associate of McDonald. The nine invited oral presentations, listed in Table 2, can be considered an assessment of the current state of the field. In particular, small-scale biological samples, matrix removal, and magnetic sorbent particles are all driving present research in SPE. This column and peer-reviewed analytical chemistry journals review the current state of SPE periodically.

Views on Future Developments in Sample Preparation
Last spring, our sister publication, The Column, featured an interview conducted by editors of LCGC with panellists from companies that exhibited at Analytica 2018 (13). The topic was to assess the trends and developments in the chromatography sector. Specifically, representatives from Biotage (Paul Roberts), CEM (Alicia Stell), Eprep (Peter Dawes), Gerstel (Oliver Lerch), Phenomenex (Matt Brusius), and UCT (Danielle Mackowsky) were asked their opinions on emerging sample preparation trends, important recent developments, obstacles to continued sample preparation developments, and the biggest accomplishments in the past year. While each of the six panellists provided perspectives from their viewpoint, common threads centred on development of faster and higher throughput techniques, automation, and accommodation of smaller samples. Since this interview is freely available on-line (http://www.chromatographyonline.com/trends-and-developments), the reader is invited to peruse this article.
Meanwhile, at about the same time, on 19–22 June, was the 20th International Symposium on Advances in Extraction Technology (ExTech) in Ames, Iowa, USA. During one feature, conference organizer Jared Anderson from Iowa State University assembled a panel that included technical experts from sample preparation vendors and academia. I was honoured to join the panel, which also consisted of Veronica Pino-Estevez (Universidad de La Laguna, Spain), Elia Psillakis (Technical University of Crete), Janusz Pawliszyn (University of Waterloo, Canada), Bruce Richter (Agilent Technologies), Dan Cardin (Entech), and Jason Herrington (Restek). An interesting mix of views, with some significant convergence, was noted. Based on the panel discussion, new developments in the near term were discussed. These include approaches for matrix removal, which differ from sample enrichment, field-based extractions and other approaches to take the extraction to the sample, and even one-size-fits-all approaches to the extraction of complex samples. PinoâEstevez and Richter discussed how separation scientists can learn from materials science. For example, Pino-Estevez opined that the preparation of core–shell magnetic materials will drive advances in magnetic-particle-based separations. Psillakis, on the other hand, offered the view that advanced, or smart, materials are not necessary for development of sample preparation. For example, she has demonstrated success in the manipulation of vacuum conditions to provide more efficient extractions and mentioned the salting-out effect in driving chemical separations. Neither of these manipulations (reduced pressure and salting out) can compete with the effect of temperature in driving separations. This led the panel, notably Psillakis, Pawliszyn, and me, to stress that what is missing-and limiting-future innovations, is the understanding of the fundamentals of extraction. For example, at its heart, all extractions involve manipulations of phase contact, solubility, diffusion, and phase separation in a thermodynamically consistent manner. Knowledge of the chemistry of analytical systems and the impact of various manipulations (such as vacuum or temperature) can influence many technologies. Pawliszyn mentioned the integration of the individual steps in an analysis scheme, modelling, optimization of mass transfer, and direct coupling to mass spectrometry as needs for the future development of sample preparation.
Pawliszyn noted the lack of industrial chemists at the ExTech conference, though it is noted that the infrequent appearance of ExTech symposia in North America (the previous ExTech in the U.S. was in the Black Hills of South Dakota nearly ten years ago, in 2009) and the plethora of specialized meetings can contribute to lower attendance. He observed a conflict between industrial adoption of new separation technology with early inventors. Similarly, claims that regulatory methods are generally developed by academics and technology manufacturers, due to budgetary and workload issues in laboratories associated with government agencies, may also minimize industrial adoption of new sample preparation technologies. Hence, the drivers for sample preparation development have shifted from being the pull of industry needs to the push of new technologies in search of meaningful applications. However, education towards a more complete understanding of solubility and phase equilibria will guide all analysts in coming up with the sample preparation approaches of the future.
References
- D.E. Raynie, LCGC Europe31(8), 437–439 (2018).
- W.J. Youden, Anal. Chem.19, 946–950 (1947).
- W.J. Youden, Biometrics3, 61 (1947).
- W.J. Youden, Mater. Res. Stand.1, 268–271 (1961).
- W.J. Youden, Statistical Techniques for Collaborative Tests. Association of Official Analytical Chemists, Washington, DC, USA, 1967.
- W.J. Youden and E.H. Steiner, Statistical Manual of AOAC. Association of Official Analytical Chemists, Washington, DC, USA, 1975.
- E. Karageorgou and V. Samanidou, J. Chromatogr. A1353, 131–139 (2014).
- R.C.C. Castells and M.A. Castillo, Anal. Chim. Acta423, 179–185 (2000).
- A.R. Mauri, M. Llobat, and D. Adria, Anal. Chim. Acta426, 135–146 (2001).
- A.G. Gonzalez and M.A. Herrador, TrAc Trend Anal. Chem.26, 227–238 (2007).
- P.D. McDonald, “James Waters and his Liquid Chromatography People: A Personal Perspective,” http://www.waters.com/webassets/cms/library/docs/wa62008.pdf.
- E.S. Bouvier, R.E. Meirowitz, and P.D. McDonald, U.S. patent 5,976,367 (1996).
- LCGC Editors, The Column14, 2–19 (2018).
Douglas E. Raynie is a Department Head and Associate Professor at South Dakota State University. His research interests include green chemistry, alternative solvents, sample preparation, high-resolution chromatography, and bioprocessing in supercritical fluids. He earned his Ph.D. in 1990 at Brigham Young University under the direction of Milton L. Lee. Raynie is a member of LCGC’s editorial advisory board. Direct correspondence about this column via e-mail to LCGCedit@ubm.com

Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.













