Liquid Chromatography Coupled with Tandem Mass Spectrometry for Clinical Applications
Special Issues
The use of mass spectrometry (MS) in clinical diagnosis goes back to the early 1970s with the application of gas chromatography (GC)–MS to the determination of a variety of biologically significant molecules. Because GC requires a certain level of analyte volatility, and since most biologically active molecules are polar, thermolabile, and involatile, elaborate extraction and derivatization protocols needed to be devised to make GC–MS useful for the analysis of clinically relevant samples. To make sample analysis less difficult by MS there had been a significant amount of R&D invested over several decades aimed at coupling high performance liquid chromatography (HPLC) with MS since HPLC is a much better separation technology than GC for polar thermolabile biologically relevant molecules. This coupling was not without significant challenges; most of the LC–MS coupling techniques that evolved during the 1970s and 1980s were not very successful, and many of those that enjoyed some widespread..
The use of mass spectrometry (MS) in clinical diagnosis goes back to the early 1970s with the application of gas chromatography (GC)–MS to the determination of a variety of biologically significant molecules. Because GC requires a certain level of analyte volatility, and since most biologically active molecules are polar, thermolabile, and involatile, elaborate extraction and derivatization protocols needed to be devised to make GC–MS useful for the analysis of clinically relevant samples. To make sample analysis less difficult by MS there had been a significant amount of R&D invested over several decades aimed at coupling high performance liquid chromatography (HPLC) with MS since HPLC is a much better separation technology than GC for polar thermolabile biologically relevant molecules. This coupling was not without significant challenges; most of the LC–MS coupling techniques that evolved during the 1970s and 1980s were not very successful, and many of those that enjoyed some widespread popularity, such as thermospray, flow-fast atom bombardment (FAB), and particle beam, are now virtually extinct. All of these techniques were quickly displaced by interfaces involving ionization techniques that separated the ionization process from the high vacuum analyzer portion of the instruments, namely atmospheric pressure ionization (API), including electrospray (ESI), ion-spray (nebulizer-assisted electrospray, ISP), atmospheric pressure chemical ionization (APCI) using a heated nebulizer, and atmospheric pressure photoionization (APPI), also using a heated nebulizer. Not until the development of API did there become a much more rapid adoption of LC–MS to the analysis of clinically important samples.
An exceptionally good review of the development of API techniques was written by Thomson (1). As the name implies, API creates ions at atmospheric pressure, outside the pristine analyzer, thereby making LC–MS very robust and relatively free of analyzer contamination. Coupling of HPLC to API-MS is trivial since the MS vacuum system is not directly involved; this relative ease-of-use permitted those researchers more familiar with biochemistry than with analytical technologies to actually use LC–MS more routinely. This caused an enormous increase in applications for LC–MS across a wide range of biologically significant molecules, including biopolymers such as polysaccharides, DNA, and proteins as well as small molecule analytes such as nucleotides, amino acids, acylcarnitines, sphingolipids, phospholipids, and biogenic amines. The vast majority of LC–MS applications for clinical research today are being run using API.
One of the features of API techniques is that it is very "soft," creating primarily intact molecular ions, which, unless very high resolution and mass accuracies are employed, are not very analytically specific. This has necessitated the use of tandem mass spectrometry, or MS-MS, to enable the analysis of trace analytes from complex biological matrices typically encountered in clinical research samples. There have been some excellent reviews on this subject (2,3).
Examples of the most prevalent uses of MS-MS in clinical research include the screening of newborns for congenital metabolic diseases such as aminoacidopathies, organic acidurias, and fatty acid oxidation disorders (4,5); multi-analyte therapeutic drug monitoring (TDM), especially for the administration of cocktail therapies involving immunosuppressants (6–8), oncology drugs (9), and anti-retrovirals (10,11); toxicant and drugs-of-abuse screening, in which samples can be screened and validated in a single run (12,13); the analysis of endogenous peptides, especially where different isoforms exist; and the analysis of steroid hormones (14). With respect to the latter, there has been a recent growing level of interest in the application of LC–MS-MS to clinical research in endocrinology to the point where the American Endocrine Society has issued a statement recommending LC–MS-MS for the determination of endogenous levels of steroid hormones such as testosterone over more traditional technologies such as immunoassays (15). The rationale for this position has been the superiority of analytical results by LC–MS-MS, especially for low levels of these analytes (16,17). The reader is referred to a recent review article on the use of LC–MS-MS for a variety of endocrinology applications (18). Despite these useful applications and the rapid growth of LC–MS-MS in clinical research, the number of LC–MS-MS systems in use in routine diagnostic laboratories is actually relatively small compared to more traditional biochemical or immunological analyzers.
For clinical research, the major advantages of LC–MS-MS over traditional technologies are the speed of assay development; the same relatively low cost-per-assay whether there is one or many analytes in a single sample; high specificity, significantly higher than immunoassays especially for small molecule analytes; and no costly analyte specific reagents (ASRs) are required. There are also some disadvantages to LC–MS-MS, such as the high capital cost of these instruments, usually with no "reagent-rental" payment options; LC–MS-MS systems are complex instruments and not trivial to learn to operate; LC–MS-MS is not sensitive enough for some important classes of analytes compared to immunoassays (such as proteins); and relatively labor-intensive or complicated sample preparation is sometimes required for trace analytes in biological matrices. Such sample preparation techniques are necessary because of the relatively high levels of proteins, salts, and lipids encountered in biological matrices, which can interfere with the ionization of analytes should they be coeluted. These disadvantages are responsible for the relatively limited uptake of LC–MS-MS into routine clinical diagnostic workflows where technicians operating instruments are normally required only to place samples directly onto the analyzers with little or no pretreatment.
Recently, significant breakthroughs in sample preparation and multidimensional chromatography have been commercially introduced that are solving many of these issues for LC–MS-MS. Specifically, turbulent flow chromatography (TurboFlow, Thermo Scientific, Franklin, Massachusetts) has enabled rapid highly automated on-line sample extraction before analysis by LC–MS-MS. Instruments such as the Thermo Scientific Aria LC–MS system and Transcend TLX turbulent flow ultrahigh-pressure liquid chromatography (UHPLC) systems (Thermo Fisher Scientific, Waltham, Massachusetts) can accommodate the direct analysis of biological samples by LC–MS-MS, often only requiring sample dilution before placing the sample onto the system. This minimizes time- and resource-consuming off-line sample preparation, which can often lead to delays in assay results or to the introduction of errors associated with manual sample handling, transfer, or aliquoting.
TurboFlow technology involves the proprietary use of large-diameter particles (~ 30 μm) with a high surface area packed into narrow-bore columns (0.5 to 1 mm i.d.) that are available in a variety of stationary phases. Samples such as dilute serum, cerebrospinal fluid, plasma, urine, and biological matrixes are injected directly onto these columns under high linear velocities, inducing turbulent-flow conditions; this is as opposed to laminar flow, considered ideal for conventional chromatography. Under turbulent flow conditions, small molecule analytes are able to diffuse into the cavities on the surface of the large particles where they are retained, depending upon the stationary phase characteristics and the properties of the analytes of interest. Proteins are too large, and the mobile-phase linear velocities too high (column residence time too low) to allow these compounds to diffuse effectively into the surface pores and be retained. Furthermore, salts are too polar to be retained; consequently turbulent flow chromatography simultaneously eliminates interferences from salts and proteins. Lipids are often retained in turbulent flow chromatography; however, judicious choice of loading and eluting mobile phases as outlined by the manufacturer eliminates interference from this class of compounds in the analysis of a wide variety of analytes including lipophilic compounds such as steroids (Figure 1). Figure 1 demonstrates the ability of turbulent flow chromatography to eliminate ion suppression. In the present experiment a 1 ng/mL solution of nandralone, an anabolic steroid, is continuously infused at 5 μL/min after the analytical HPLC column while a negative control serum sample and a water-blank are processed by a multidimensional LC–MS-MS system. There is no perturbation of the nandralone response during its expected retention time window (shaded area). The result for the negative control serum sample is essentially identical to the water blank, confirming a complete absence of any ion-suppressing matrix components from equine serum during this critical retention period. These data were part of a thorough study on the use of the turbulent flow chromatography system for the determination of the anabolic androgenic steroids trenbolone, boldenone, nandrolone, testosterone, and stanozolol in equine serum (19). Turbulent flow chromatography greatly simplifies sample preparation, allowing LC–MS-MS to fit more effectively into routine workflows in laboratories doing clinical research.

Figure 1
Besides sample handling, one of the other major issues with LC–MS-MS in clinical research as noted earlier is the high capital cost of instruments. The LC–MS-MS experiment is often quite inefficient as visualized in Figure 2 top trace, where the MS-MS analyzer is acquiring meaningful data only 25% of the time; in this case, most of the run-time is actually involved in waiting for the analytes of interest to emerge from the column or for re-equilibration. The Transcend TLX-4 system can increase duty cycle and throughput by up to 400%, increasing the overall efficiency of the LC–MS-MS system and contributing significantly toward return on investment (Figure 2, bottom). The turbulent flow systems are also capable of utilizing UHPLC; when combined with < 2 μm particle size, analytical columns can further shorten the chromatographic time scale, increasing throughput and making multiplexing that much more efficient.

Figure 2
Fentanyl in Urine
Many applications now exist demonstrating the workflow simplification and increased throughput using multidimensional multiplexed LC–LC–MS-MS systems. Laboratories with high sample throughput requirements are especially receptive to this technology if the advantages also include no compromises in data quality. One such method was developed for the therapeutic drug monitoring (TDM) of a prescription analgesic (21). Much of the TDM of analgesic drugs is currently being done using GC–MS, which is the gold standard for such applications. Occasionally, certain pain medications are better analyzed using liquid introduction techniques like LC–MS-MS because their properties, such as thermolability or high polarity, make them incompatible with GC introduction, or they may be dosed at low levels, making the higher sensitivity of LC–MS-MS more suitable. The TLX-4 system used for this study was configured with a TSQ Quantum Access triple-quadrupole MS-MS system (Thermo Fisher Scientific).
Fentanyl is a potent opioid analgesic that is 80 times stronger than morphine used to treat chronic pain. Because of its potency, it is a candidate for TDM to ensure compliance and that proper dosing regimes are adhered to.
The method involved the dilution of urine samples with the addition of the internal standards fentanyl-d5 and norfentanyl-d5, followed by vortex and centrifuge before analysis. Norfentanyl is one of the major metabolites of fentanyl and both are excreted in urine. The two analytes and internal standards were detected by LC–MS-MS using multiple reaction monitoring (MRM), in which the MS-MS system selects molecular ions of each analyte with the first MS analyzer and monitors one or more product ions with the second MS. The MRM channels were 337→188 and 337→105 for fentanyl, 342→188 and 342→105 for fentanyl-d5, 233→177 and 233→84 for norfentanyl and 238→155 and 238→84 for norfentanyl-d5. Ionization was by heated electrospray ion source (H-ESI). Calibrators ranging from 0.5 to 200 ng/mL were created in negative control urine and multiple calibration curves generated per sample batch. Two MRM channels per analyte were used, and their ratios had to be maintained within stringent intensity ratio criteria to ensure specificity as defined by WADA.
The total HPLC run time per sample was 4.3 min; a typical MRM trace is shown in Figure 3. Each turbulent flow cycle requires less time than the HPLC method. After each cycle, a plug of cleaned-up analyte is sent to its respective HPLC column for analysis; however, only the critical 1.3-min interval, when the analytes of interest emerge from the HPLC, is actually sampled by the MS-MS. S/N values were acceptably high as indicated, allowing quantification within what was considered to be the therapeutic range. The Aria software automatically uses all four turbulent flow channels with their respective HPLC columns in a staggered manner, increasing throughput and duty cycle by over 300%. Hundreds of samples can be run on a single four-channel turbulent flow system each day.

Figure 3
Calibration curves for each of the separate turbulent flow channels were essentially superimposable, typically demonstrating correlation coefficients (R2 ) of > 0.99 and CVs, of between 5% and 15% for large sample sets over lengthy time periods (up to 2 weeks). Besides the increased productivity of the analyzer, sample preparation was greatly simplified compared with the GC–MS method — no extraction was required; no derivatization was required; no evaporation or re-extraction steps were needed; a 15-step method for GC–MS was replaced by a greatly simplified five-step method for LC–MS-MS; tubes needed to be labeled only once for the turbulent flow method compared with four times per sample for GC–MS; one four-channel TurboFlow–MS system was equivalent in output to four or five GC–MS systems for this analyte; but most importantly, the higher sensitivity and specificity afforded by LC–MS-MS for fentanyl increased confidence in the analytical results.
Testosterone in Plasma
Testosterone is one of the most challenging analytes in endocrinology clinical research, especially when evaluating the low testosterone levels found in hypogonadal males, in women, and in prepubertal children. Testosterone is a major androgenic hormone crucial to the development of male reproductive organs and masculine characteristics in adults. In females, its main role is as an estrogen precursor. Testosterone measurements are frequently used for the diagnosis and management of male hypogonadism and pubertal delay. In adult women, excess testosterone production results in varying degrees of virilization, including hirsutism or infertility. Mild-to-moderate testosterone elevations are usually asymptomatic in males, but can cause distressing symptoms in females.
For adults, the normal values for testosterone are about 300–1000 ng/dL for males and 20–80 ng/dL for females. Problems occur with immunoassays below the sub-100 ng/dL level; in recognition of this, the Endocrine Society and the CDC have recently started an initiative, recommending that "in the absence of other information, direct immunoassays (those performed on whole serum or plasma) perform poorly at low testosterone concentrations (i.e., women, children, and hypogonadal men) and should be avoided" (15,22).
The requirements for increased precision, accuracy, and sensitivity have led many clinical researchers to use isotope dilution (ID) LC–MS-MS for the determination of reference ranges for subjects exhibiting symptoms indicative of these lower testosterone concentrations. Traditionally, steroid hormones such as testosterone were extracted from plasma or serum using liquid–liquid extraction (LLE) methods. Even after LLE, extracts contain considerable matrix (such as phospholipids), requiring relatively long LC–MS-MS run times. Compared with immunoassays, LC–MS-MS methods using LLE impose punishing time and resource requirements on labs wishing to run many samples.
Scientists at the larger reference laboratories (23) have recently begun to use the Aria and Transcend multidimensional LC–MS-MS systems to eliminate complicated off-line sample preparation methods like LLE and replace them with on-line extraction methods (Table I). These researchers have observed that on-line sample cleanup typically reduces prep time by about a factor of 6 over LLE and reduces sample preparation costs by 70–80%. Furthermore, interference from proteins, salts, and phospholipids can be effectively eliminated and LC–MS-MS run times reduced significantly with multidimensional LC–MS-MS.
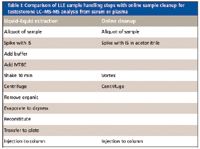
Table I: Comparison of LLE sample handling steps with online sample cleanup for testosterone LCâMS-MS analysis from serum or plasma
In the present example, a 0.1-mL aliquot of serum was diluted with 0.25 mL acetonitrile containing the internal standard, mixed, centrifuged, and placed onto the Aria TLX system for analysis. The MS-MS instrument used was a Thermo Scientific TSQ Quantum Ultra with IonMax APCI ion source and run in the positive ionization mode where the vaporizer temperature was 525 °C and the capillary temperature 350 °C. Calibration standards were prepared in 5% BSA in 0.01 M PBS (pH 7.4) at the following concentrations: 5, 10, 20, 50, 100, 200, 500, 1000, and 2000 ng/dL.
The sample injection volume was 100 μL. The cleanup column was a 4 mm × 2 mm i.d. C18 column; the sample was loaded with 100% water, then washed with 10% methanol and eluted onto the analytical column with 80% methanol at 150 μL/min. On its way to the analytical column, the cleanup column effluent was diluted with 600 μL/min of 20% acetonitrile and loaded onto the column (Hypersil Gold C18; 33 × 4.6 mm 3 μm). The sample was finally eluted into the MS-MS with 1.0 mL/min of 50% acetonitrile. The total analytical LC–MS-MS run time was just over 4 min where the testosterone is eluted at around t = 2.6 min. The total analysis time per channel, including on-line extraction, was 6.02 min where the MS-MS system monitors each channel's effluent for about 1.2 min centered around testosterone's retention time (Figure 4).

Figure 4
The testosterone MRM transitions were 289.2→97.1 and 289.2→109.1; the internal standard was testosterone-d3 with MRM transitions 292.2→97.1 and 292.2→109.1 using a dwell of 100 ms per channel. The method precision was evaluated by analyzing subject sample pools at concentrations of 20, 50, 140, and 1000 ng/dL. Intra-assay variability was determined by processing and analyzing 20 replicates of the lowest two QC sample pools. Interassay variability was determined by processing and analyzing two replicates of each QC sample pool in six different batches. Intra-assay and interassay precision results are displayed in Table II as % CV.
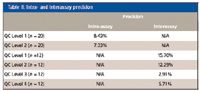
Table II: Intra- and interassay precision
Multiplexed on-line cleanup and analysis with the TLX-4 system permitted the continuous staggered injection of a diluted serum sample every 1.3 min, providing a throughput of over several hundred samples per instrument-day. Calibration curves for each channel were similar to that of Figure 5, showing good linearity from 5 ng/dL to 2000 ng/dL and typical correlation coefficients of R2 > 0.99.
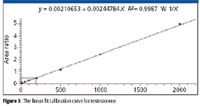
Figure 5
This testosterone method provided a fully automated system capable of analyzing dilute serum samples with no manual sample preparation steps. Multiplexing using the four-channel TLX-4 system increased throughput, allowing for duty cycle and efficiencies of about 400% over single channel operation and very high throughput for this challenging analyte.
Traditionally, clinical laboratories have used systems that required extensive sample preparation and derivatization. By adding an LC-LC–MS-MS technique for these applications, scientists doing clinical research can not only benefit from the sensitivity and confidence of MS-MS data, but also reduce the sample preparation steps such as extraction, derivatization, and evaporation. This is particularly true for lipophilic analytes such as steroids and vitamin D and its hydroxylated metabolites, described below.
Vitamin D in Serum
Thermo Fisher Scientific developed a quantitative method for analyzing 25-hydroxy-vitamin D3 and 25-hydroxy-vitamin D2 using the TLX-4 TurboFlow system (24). The method enables an online extraction of biological samples. As demonstrated earlier, online sample extraction has the potential to greatly reduce the sample preparation procedures and therefore a potential source of human error and cost. The combination of turbulent flow chromatography and LC–MS-MS enables clinical research laboratories to provide even faster turnaround times as well as more accurate and reliable analytical results.
The level of vitamin D in serum is measured by analyzing its major metabolites. Among the vitamin D metabolites, 25-OH-D has the highest concentration and longest half-life in blood. It is considered to be the best indicator of vitamin D concentration in the blood. The MS analysis for this method was carried out on a TSQ Quantum Ultra MS-MS system. Table III lists the SRM transitions and their parameters.
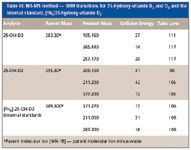
Table III: MS-MS method - SRM transitions for 25-hydroxy-vitamin D2 and D3 and the internal standard, [2H6]25-hydroxy-vitamin D3
Turbulent flow methods reduce matrix effects and allow users to inject biological fluids directly into the LC–MS-MS system without further sample handling. Before injection into the turbulent flow system, the 25-hydroxy-vitamin D samples require protein precipitation due to the strong protein binding, thus releasing the analyte for analysis. Injecting the precipitated biological samples into the four-channel turbulent flow system ensures the removal of the interferences, or potential interferences, which may affect method performance when injecting thousands of biological samples, including salts, phospholipids, and residual proteins. This is a significant advantage in laboratories where sample volumes are high, making extensive manual sample preparation impractical. Figures 6 and 7 show the linear calibration curves, both with R2 values better than 0.99 for 25-OH vitamin D3 and D2 in bovine plasma, respectively. Table IV shows a data summary.
Figure 8 shows the representative lower limit of quantitation (LLOQ) chromatogram of 4 ng/mL 25-OH-D3, internal standard, and 25-OH-D2 in bovine plasma.
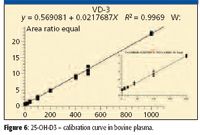
Figure 6
The four-channel turbulent flow–MS system delivered fast and efficient quantitative results with negligible ion suppression and low chemical noise. Throughput was as high as a sample approximately every 1.2 min when analyzed in a multiplexed fashion. The mass spectrometer has available a unique H-SRM mode (high resolution precursor ion mass selection, 0.2 u FWHH), allowing for much higher analyte specificity, and as a result, both LLOQ and LLOD were improved. Wide dynamic range of 4–1000 ng/mL (10–2500 nmol/L) was reported. Similar to the above studies, the % CV values for both neat samples and plasma samples meet the bioanalytical method validation criteria for GLP laboratories, which require less than 20% for LLOQ and less than 15% for all the other points on the calibration curve.
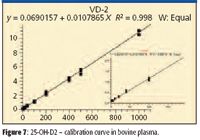
Figure 7
As shown, turbulent flow chromatography can provide a fast, robust, and sensitive analytical method for quantitative analysis with minimal sample pretreatment for a wide variety of endogenous biologically important compounds. This method can be multiplexed using a four-channel turbulent flow system, which will provide a result about every 1.0 to 1.5 min, or over 40 per hour. One mass spectrometer with a four-channel turbulent flow system in multiplexing mode can analyze four times as many samples as a single LC-LC–MS-MS system. These benefits offer significant advantages to clinical research and reference laboratories looking to improve efficiencies and reduce operational costs. For example, data recently presented indicated a cost reduction of over 70% was realized for this assay by switching from radio immunoassay to the four-channel turbulent flow LC–MS-MS system (25).
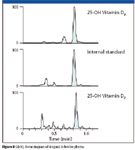
Figure 8
Conclusion
LC–MS-MS is emerging as a complementary method to traditional methodologies used for clinical applications. Reduced sample preparation and high-throughput capabilities as demonstrated here can provide significant benefits to clinical scientists conducting routine analyses. Although the initial capital investment for LC–MS-MS instrumentation is substantial compared to traditional techniques, the operational costs are low and the increased flexibility, sensitivity, and specificity are valuable. The addition of turbulent flow and multiplexing capabilities enables a high degree of automation and simplicity in sample handling as well as increased efficiency. As such, the combination of these technologies is expected to expand rapidly in the future development of clinical laboratory services by overcoming the main obstacles to implementation, namely cost and ease-of-use. As scientists focus on more complicated challenges that can be solved efficiently by adding LC–MS-MS to their arsenal of techniques, turbulent flow and multiplexing on-line sample preparation systems will undoubtedly play an increasing role.
Jeffrey Zonderman, Director Clinical and Toxicology LC/MS, Thermo Fisher Scientific, Franklin, Massachusetts and Bori Shushan, Ph.D., CSO, Clinical Mass Spec Consultants, Toronto, Ontario.
References
(1) B.A. Thomson, J. Am. Soc. Mass Spectrom . 9, 187–193 (1998).
(2) K.C. Dooley, Clin. Biochem . 36, 471–481 (2003).
(3) M. Vogeser and C. Seger, Clin. Biochem . 41 649–662 (2008).
(4) M.S. Rashed, et al., Clin. Chem . 43, 1129–1141 (1997).
(5) D.H. Chace, T.A. Kalas, and E.W. Naylor, Clin. Chem . 49, 1797–1817 (2003).
(6) M. Constanzer, C. Chavez, and B. Matuszewski, J. Chromatogr., B 666, 117–126 (1995).
(7) P.J. Taylor, A. Jones, G.A. Balderson, S.V. Lynch, R.L.G. Norris, and S.M. Pond, Clin. Chem. 42, 279–285 (1996).
(8) Z. Yang, Y. Peng, and S. Wang, Clin. Appl. Immunol. Rev. 5, 405–430 (2005).
(9) L. Lennard, Br. J. Clin. Pharmacol. 52, 75S–87S (2001).
(10) D.J. Back, S.H. Khoo, S.E. Gibbons, and C. Merry, Br. J. Clin. Pharmacol. 52, 89S–96S (2001).
(11) J. Gu and S.J. Soldin, Clin. Chim. Acta 378(1–2),222-224 (2007)
(12) H.H. Mauerer, Anal. Bioanal. Chem . 388, 1315–1325 (2007).
(13) P. Marquet, et al., Clin. Chem. 52(9), 1735–1742 (2006).
(14) J.P. Holst, S.J. Soldin, R.E. Tractenberg, T. Guo, P. Kundra, J.G. Verbalis, and J. Jonklaas, Steroids 72(1), 71–84 (2007).
(15) W. Rosner, R.J. Auchus, R. Azziz, P.M. Sluss, and H. Raff, J. Clin. Endocrinol. Metab . 92, 405–413 (2007).
(16) J.P. Holst, O.P. Soldin, T. Guo, and S.J. Soldin, Clin. Lab Med. 24(1), 105–118 (2004).
(17) T. Guo, M. Chan, and S.J. Soldin, Arch. Pathol. Lab Med. 128(4), 469–475 (2004).
(18) M. Vogeser, Exp. Clin. Endocrinol. Diabetes 115, 559–570 (2007).
(19) B.C. Moeller and S.D. Stanley, A Rapid Quantitative Method for Multiple Anabolic Steroids in Equine Serum by Turbulent Flow Chromatography Tandem MS-MS, K.L. Maddy Equine Analytical Chemistry Laboratory, CAHFS, University of California–Davis, presented at the June 2008 annual meeting of the American Society of Mass Spectromery in Denver, CO (poster MPI190).
(20) J.M. Lacey, M.J. Magera, J.M. Di Bussolo, F. Lorey, S. Tortorelli, D. Gavrilov, P. Rinaldo, and D. Matern, Development of a Method for the Determination of Congenital Adrenal Hyperplasia (CAH) by Turbulent Flow Chromatography and LC-MS/MS, Presented at the American Association of Clinical Chemistry Annual Meeting, Poster D-135, San Diego CA, July 18, 2007.
(21) D. Crouch, The Analysis of Fentanyl and Norfentanyl Using TurboFlow Column Analyte Isolation and Multiplex-HPLC/MS/MS, Ameritox, Midland, TX, Presented at the 60th Annual Meeting of the American Association of Forensic Scientists, February 19, 2008, Washington DC.
(22) The Endocrine Society and CDC Collaborate to Standardize Testosterone Assays for Clinical Practice. http://www.endo-society.org/news/press/ (accessed: April, 2008).
(23) R.J. Singh, J.L. Bruton, and T. Rezai, Quantitative Analysis of Testosterone in Serum by LC-MS/MS, App Note 429, Thermo Fisher Scientific, San Jose, CA, 2008.
(24) A. Zhang, R. Kane, and F. Espourteille, A Quantitative, Efficient Method for Analysis of 25-Hydroxy-Vitamin D3 and 25-Hydroxy-Vitamin D2 Using TurboFlow Technology. Application Note 416: Thermo Fisher Scientific, San Jose, California, 2008.
(25) Dr. Sihe Wang, Cleveland Clinic, personal communication, 2008.

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









