The Impact of Fast Chromatography and Its Effects on Mass Spectrometry
Special Issues
For many years, and after several notable failures, many researchers were convinced that it was impossible to design a quadrupole time-of-flight (qTOF) mass spectrometer that was able to retain its ability to perform the high-resolution measurements necessary for definitive molecular formula determination of unknowns. Conventional wisdom indicated that there were many reasons (for example, temperature stability, ion diffusion, and ion loss on grids of reflectrons) that would make it impossible to improve resolution of these types of instruments. Figure 1 shows a schematic of an instrument designed for high-resolution measurements with fast chromatography (Maxis UHR-TOF mass spectrometer, Bruker Daltonics, Billerica, Massachusetts). The instrument includes an ion chiller, a series of ion refocusing operations, a single reflectron, and temperature control of the overall flight tube of the instrument.
For many years, and after several notable failures, many researchers were convinced that it was impossible to design a quadrupole time-of-flight (qTOF) mass spectrometer that was able to retain its ability to perform the high-resolution measurements necessary for definitive molecular formula determination of unknowns. Conventional wisdom indicated that there were many reasons (for example, temperature stability, ion diffusion, and ion loss on grids of reflectrons) that would make it impossible to improve resolution of these types of instruments. Figure 1 shows a schematic of an instrument designed for high-resolution measurements with fast chromatography (Maxis UHR-TOF mass spectrometer, Bruker Daltonics, Billerica, Massachusetts). The instrument includes an ion chiller, a series of ion refocusing operations, a single reflectron, and temperature control of the overall flight tube of the instrument.
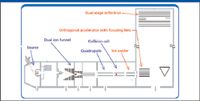
Figure 1: Schematic of a high-resolution TOF mass spectrometer (Maxis UHR-TOF mass spectrometer, Bruker Daltonics).
Evaluating Method Performance with Fast Chromatography of Small Molecules
To evaluate the performance of the fast chromatography–MS method for use in molecular formula determinations, a series of five unrelated drug compounds was studied. These compounds were chromatographed using a reversed-phase column over a 30-s elution gradient. The results of the initial studies are presented in Figure 2.

Figure 2: Separation and measurement of drug compounds with fast chromatography.
As can be seen, the method was able to achieve baseline separation of the five compounds in 30 s. In addition, the peak widths, when measured at half height, were all less than 1 s.
Figure 3 is a closer examination of the fourth peak in the compound series, oxybutynin. The left half of the figure shows the extracted ion chromatogram, demonstrating that even with a 1-s peak, over 20 data points were taken. This leads to the high levels of both mass accuracy (0.8 ppm) and resolution (>40,000) shown in the right half of the figure. Other peaks showed similar results.

Figure 3: Close examination of a single fast chromatography peak.
Evaluating Method Performance with Fast Chromatography of Peptides and Proteins
As many research groups are focusing more and more on protein and peptide analysis, the method was also tested for its ability to analyze proteins and peptides effectively. Figure 4 illustrates the data obtained when measuring isobaric peptides. The data shown here indicate that even with isobaric peptides, the method is able to differentiate these peptides very clearly, even when the molecular weight difference is only 50 mDa. Finally, a profile of a whole cell lysate was obtained to determine the number of proteins that could be identified. The results of this experiment are shown in Figure 5.

Figure 4: Resolution of isobaric peptides.
The high-resolution MS method enabled identification, with a high degree of certainty, of a very large number of proteins, even in a complex sample such as a cell lysate. Not only were the number of proteins identified very large, but the overall accuracy of identification was also very good. The high-resolution capability demonstrated here would be extremely useful for rapid profiling of a series of proteins or peptides in a biomarker study, for instance.

Figure 5: MS analysis of E. coli whole cell lysate.
Conclusion
Today's analytical chemistry environment demands the deployment of more sophisticated methods and instrumentation to keep pace with the profound changes in separation techniques being adopted by many laboratories. Two of the most important tools for the future will be fast chromatography and MS. The method described in this article represents a successful combination of high-resolution MS with fast chromatography.
Darwin Asa is marketing manager with Bruker Daltonics, Billerica, Massachusetts.

Determining Enhanced Sensitivity to Odors due to Anxiety-Associated Chemosignals with GC
May 8th 2025Based on their hypothesis that smelling anxiety chemosignals can, like visual anxiety induction, lead to an increase in odor sensitivity, a joint study between the University of Erlangen-Nuremberg (Erlangen, Germany) and the Fraunhofer Institute for Process Engineering and Packaging (Freising, Germany) combined behavioral experiments, odor profile analysis by a trained panel, and instrumental analysis of odorants (gas chromatography-olfactometry) and volatiles (gas chromatography-mass spectrometry).
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









