Liquid Chromatography of Carbohydrates in Human Food and Animal Feeding Stuffs
Special Issues
Food for human consumption and animal feedstuffs contain a variety of mono-, di-, oligo-, and polysaccharides with different functions. In this article specific liquid chromatographic (LC) systems (column, mobile phase, and detector), which are used to determine different carbohydrates in food and feed matrices, are described.
Food for human consumption and animal feedstuffs contain a variety of mono-, di-, oligo-, and polysaccharides with different functions. In this article specific liquid chromatographic (LC) systems (column, mobile phase, and detector), which are used to determine different carbohydrates in food and feed matrices, are described. Cation-exchange columns with different cation counter ions (Na+, Ag+ , Ca++, and Pb++) in combination with a refractive index detector have been widely used for the analysis of mono-, di-, and oligosaccharides for many years. Currently high performance anion exchange chromatography with pulsed amperometric detection (HPAEC–PAD) is often applied in these analyses. Hydrophilic interaction liquid chromatography (HILIC) with mostly aqueous organic mobile phases combined with mass spectrometric detection is a very powerful tool for both the qualitative and quantitative analyses of complex carbohydrates.
Carbohydrates are one of the most abundant distributed constituents in living nature. Together with fat and protein, carbohydrates are a major food constituent. Food and feed contain a broad variety of different carbohydrates as mono-, di-, oligo-, and polysaccharides. In Figure 1 an overview is given of the different carbohydrates that can be present in food and feed. The different categories of carbohydrates have a variety of functions in food products, such as (1) a source of energy, (2) a sweetener, (3) for viscosity and texture, (4) a fat replacer, (5) dietary fiber, and (6) as a prebiotic. The content of the different carbohydrates in our food is of course related to their functionality. For example, digestible carbohydrates such as sugars, starch, and malto-oligosaccharides, and which are important for the energy content of the food product, are usually major constituents with concentration levels ranging from a very small percent to over 50%. Alternatively, carbohydrates that are important for the viscosity and texture of a food product are normally present at low concentration levels, ranging from ≤ 0.1% (for carrageenan) to about 5% (for a starch-based thickener). Dietary fibers (by EU [1] and Codex definition [2] non-digestible carbohydrates with a DP≥3) are mainly present in vegetables, fruits, nuts, and cereal products. Meat, milk, and eggs do not contain dietary fiber. Usually the dietary fiber content in food products ranges from 0–10% depending on the product. According to the Food and Agriculture Organization of the United Nations (FAO) definition of 2007 (3), prebiotics are non-viable food components that confer a health benefit on the host associated with modulation of the microbiota in the intestinal track. Common prebiotics include inulin, fructo-oligosaccharides (FOS), galacto-oligosaccharides (GOS), soya-oligosaccharides, xylo-oligosaccharides, pyrodextrins, isomalto-oligosaccharides, and lactulose.
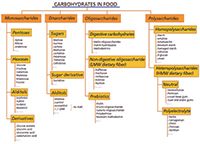
Figure 1: Schematic overview of the different carbohydrates in human food and animal feed.
Both qualitative and quantitative characterization of the different carbohydrates present in food and feed are very important to ensure the correct labeling of the product concerning the digestible carbohydrates content (part of the energetic value), dietary fiber, and prebiotic content.
In addition, the carbohydrate characterization (both qualitative and quantitative) of raw materials for manufacturing the different (non starch polysaccharide) food ingredients is essential for a good food manufacturing process.
Liquid Chromatography of Carbohydrates
Detectors
Liquid chromatography (LC) is a very well established technique for the separation and determination of carbohydrates. The bottleneck in the LC analysis of carbohydrates is the detection system. Because carbohydrates do not show a significant radiation absorption in the normal UV and visible range, UV–vis detectors are not appropriate in carbohydrate analyses unless they are derivatized with UV-adsorbing substituents. The most applied LC detectors, particularly in routine analysis, are the refractive index (RI) detector in combination with isocratic separations, the pulsed amperometric detector (PAD), and the evaporative light scattering detector (ELSD), which can both be applied with gradient elution LC systems. In addition, mass spectrometric (MS) techniques are becoming more commonly applied for carbohydrate characterizations in combination with LC.
Liquid Chromatographic Systems
Various LC systems have been described in the literature. The choice of the chromatographic system depends upon the required level of structural details (for example, total monosaccharides or separation between the monosaccharides [glucose, galactose, mannose, and fructose]), type of glycosidic bonding (α, β, or 1à4, 1à1, or 1à6), concentration level of the carbohydrate, and, of course, the sample matrix. In this mini review we will discuss three chromatographic systems: Cation-exchange chromatography; high performance anion-exchange chromatography; and hydrophobic interaction chromatography. These LC techniques are very important for the analysis of carbohydrates in food and feed.
Cation-Exchange Chromatography with RI Detection
For a long time LC separations of carbohydrates have been performed on strong cation exchange resins in the Ca++ form at elevated temperatures, applying an aqueous solution of 50 ppm calcium ethylenediaminetetraacetic acid (Ca-EDTA) as the mobile phase and RI detection (4). For all carbohydrates the refractive index increment (dn/dc) has the same value, which means that the sensitivity of the RI detector is the same for all carbohydrates irrespective of DP value or monosaccharide composition. This facilitates the quantitation of unknown carbohydrate peaks in the chromatograms. A severe drawback of RI detectors is that they can only be applied in combination with isocratic separations.
At room temperature the mutarotation of many reducing sugars is low. As a result, the separation of a mixture of carbohydrates at room temperature on a cation-exchange column will result in a needlessly complicated chromatogram with double peaks or peaks with shoulders because of the (incomplete) separation of the α- and β-anomers of the respective sugars. Increasing the temperature increases the mutarotation by a factor of approximately 2.5 for every 10 °C rise in temperature. As a result of fast mutarotation at elevated temperatures, the α- and β-anomers of the respective carbohydrates elute together in one relatively sharp peak (5). Mutarotation is also strongly catalyzed by high pH values in alkaline solutions. This alkaline catalytic effect on the mutarotation is applied in high performance anion-exchange chromatography, which will be discussed later.
The separation mechanism is twofold: Firstly, it is based on a complex formation between the hydroxyl groups of the carbohydrates and the immobilized Ca++ ions on the resin (ligand exchange mechanism); and secondly, it is based on size-exclusion effects.
The complex formation between de hydroxyl groups in the carbohydrates and the immobilized counter ions in the cation-exchange resin depends on the conformation of the hydroxyl groups (equatorial or axial) and the carbohydrate itself (chair or boat conformation), and on the specific cation counter ions. H+, Na+, K+, Ag+, Ca++, or Pb++ are used as counter ions. Na+ and K+ form no complexes with the hydroxyl groups in the carbohydrates. Applying a column in the Na+ or K+ form instead of a column in the Ca++ form results in a loss of selectivity for the different monosaccharides and polyols. Almost all monosaccharides and polyols co-elute with each other. Columns in the Na+ or K+ form are mostly applied for the separation of (malto) oligosaccharides. For the separation of different monosaccharides, cation-exchange columns in the Pb++ form are frequently applied. As a result of the differences in structural conformation of the hydroxyl groups in the various monosaccharides, the respective monosaccharides form complexes with the immobilized counter ions in the cation exchange resin with different stability constants, resulting in different retention times of the sugars eluting from the chromatographic column.
The size-exclusion mechanism of the chromatographic separation is strongly affected by the degree of divinylbenzene cross-linking of the polysterene matrix of the cation-exchange resin. Increased cross-linking decreases the pore size in the resin and therefore the size-exclusion range of the column. A linear relationship exists between the retention time and the logarithm of the molecular weight of the eluted malto-oligosaccharides, using a cation exchange column. When applying a 4% cross-linked cation-exchange resin (in the Na+ form), the size-exclusion effect ranges from DP1 up to about DP7 or DP8 for malto-oligosaccharides. Applying a degree of cross-linking of 8% decreases the size-exclusion range from DP1 to about DP3 (Figure 2). The benefit of a relatively high degree of cross-linking is that it results in a more rigid column which resists higher pressures and therefore can be operated at higher flow rates, making faster chromatographic run times possible.

Figure 2: The effect of cross-linking the cation-exchange resin on the size-exclusion performance of the separation. (a): a 4% cross-linked polystyrene divinylbenzene cross-linked cation-exchange column (REZEX RNO oligosaccharide column in Na+ form). (b): a 8% cross-linked polystyrene divinylbenzene cross-linked cation exchange column (REZEX RNM carbohydrate column in Na+ form). Mobile phase: Water at 90 °C; Column temperature = 90 °C, RI detection.
Cation-exchange chromatography in combination with RI detection is still a good technique for analyzing simple mixtures of mono-, di-, and tri-saccharides in relatively clean sample matrices such as fruit juices and beverages. Various cation exchange columns with different specifications (for example, particle size, degree of cross-linking, and counter ions) can be commercially obtained from different manufacturers.
High Performance Anion Exchange Chromatography With Pulsed Amperometric Detection (HPAEC–PAD)
The hydroxyl groups of carbohydrates are weak acids and can be ionized under strong alkaline conditions (pH>12), making it possible to achieve anion-exchange separation of carbohydrates using stong alkaline mobile phases. High performance anion-exchange chromatography with pulsed amperometric detection (HPAEC–PAD) is a very powerful LC system in the field of carbohydrate analyses.
At the beginning of the 1980s, Dennis Johnson and co-workers at Iowa State University (5,6) developed the triple pulse amperometric detector for carbohydrates. This amperometric detection system was particularly designed for the detection of carbohydrates in strong aqueous alkaline solutions (0.1 M NaOH). The major advantage of this detector lies in its high sensitivity and high selectivity for carbohydrates. Because of these properties, a less intensive sample cleanup is needed prior to the chromatographic determination of the carbohydrates when compared with the use of RI detectors. The detector can also be applied very well in combination with gradient elution LC systems. However, a drawback of this detector is that, in contrast to the RI detector, the detector sensitivity differs for the different carbohydrates. The pH of the mobile phase in the detector flow cell is of significant influence on the detector sensitivity. This necessitates the preparation of calibration curves for all carbohydrates in every application, applying the specific chromatographic conditions as used in that application.
As mentioned previously, the strong alkaline mobile phase also catalyzes the mutarotation of the reducing carbohydrates very efficiently. Using a strong alkaline mobile phase at room temperature speeds up the mutarotation in such a way that once again the α- and β-anomers of the respective carbohydrates elute together in one sharp peak.
The mobile phase composition in HPAEC–PAD significantly affects both the selectivity of the separation and the sensitivity of the amperometric detection system. Mostly (ternary) gradient elution profiles are applied with water, with 0.1 M sodium hydroxide and 0.5 M sodium acetate applied as the solvent.
In the last 25 years, HPAEC–PAD has established itself as one of the major tools for carbohydrate analyses.
Numerous different applications have been developed, and are described in the literature and summarized in review papers (8,9). With a fine-tuning of the applied ternary gradient, very complex separations can be achieved.
Eurofins Carbohydrate Competence Centre has developed and implemented numerous HPAEC–PAD applications. The application presented in Figure 3 deals with the identification and quantitation of approximately 20 different mono-, di-, and oligosaccharides present in an infant milk powder formula. In this formula three different types of oligosaccharides are present: a series of malto-oligosaccharides ranging from DP1 to DP8 (glucose up to maltaoctaose); a series of short chain fructo-oligosaccharides ranging from DP3 to DP7; and a series of short-chain inulins with end-standing glucose units, including sucrose, ranging from DP3 to DP8. The chromatographic separation of this complex matrix is completed in about 40 min.
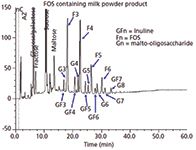
Figure 3: HPAECâPAD analysis of the carbohydrate composition of an infant formula containing malto-oligosaccharides (maltose up to maltoheptaose, G2âG7), short chain fructo-oligosaccharides (FOS, F3âF6), and short chain fructo-oligosaccharides with an end-standing glucose unit (including sucrose) GF2âGF7). CarboPak PA1 column with a 0.1 M NaOH/0.5 M NaAc/water gradient elution profile.
Another application concerns the characterization of the side chain lengths in amylopectins in native and modified starches (Figure 4). Amylopectins in starches of different botanical sources (for example, potato, wheat, and maize) show different side chain length distributions. A characteristic fingerprinting of the side chain length distribution is obtained by first applying an enzymatic debranching of the amylopectin followed by HPAEC–PAD characterization of the obtained set of amylopectin side chains. This is demonstrated in Figure 4 for a native potato starch sample. The dip in the peak heights at DP8 is more or less characteristic for native potato amylopectins. The fingerprinting for the amylopectin chain length distribution of wheat starch shows mostly a binominal distribution.
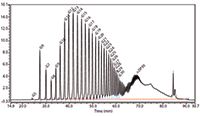
Figure 4: HPAECâPAD fingerprint of the side-chain length distribution of an enzymatically de-branched potato starch amylopectin. CarboPak PA1 column with a 0.1 M NaOH/0.5 M NaAc/water gradient elution profile.
Recently the galactosylsucrose family of oligosaccharides has become of great interest in food analyses. This interest is particularly concerned with the tri-saccharide raffinose, the tetra-saccharide stachyose, and the penta-saccharide verbascose. These oligosaccharides are present in vegetables as peas, beans, and lentils. Their content ranges from about 5% to 9% in the dry product. The galactosylsucroses are not digested in the human small intestine. They enter the colon where they are fermented by the gas-producing intestinal microflora, leading to flatulence. The vegetables contain also the usual mono- and disaccharides as glucose, fructose, sucrose, and sometimes maltose. Therefore in the galactosylsucrose HPAEC–PAD application it was necessary to achieve a good chromatographic separation between glucose, fructose, sucrose, maltose, raffinose, stachyose, and verbascose (Figure 5). Of particular note is the retention time of the disaccharide maltose: It elutes between stachyose (tetrasaccharide) and verbascose (pentasaccharide).
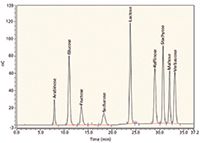
Figure 5: Chromatogram of the calibration standards in the HPAECâPAD application of the quantitative determination of galactosylsucroses in vegetables and foods using arabinose as the internal standard. CarboPak PA1 column with a 0.1 M NaOH/0.5 M NaAc/water gradient elution profile.
HPAEC–PAD is becoming more and more a standard tool for the routine analyses of carbohydrates in food and feed matrices. This is emphasized by the fact that HPAEC–PAD is also used today in both ISO (10) and CEN (11) protocols for the analysis of carbohydrates in human and animal nutrition.
Hydrophilic Interaction Liquid Chromatography
Hydrophilic interaction liquid chromatography (HILIC) can be considered as a variant of normal-phase chromatography: A hydrophilic stationary phase is applied in combination with a mostly organic mobile phase and elution is usually performed by increasing the water content. The technique was introduced in 1990 by Alpert (12) and is suited to the analysis of carbohydrates. HILIC is often combined with evaporative light scattering detection (ELSD) (13). An advantage of the ELSD over the RI detector is that it also can be used with a gradient elution. HILIC in combination with MS appears to be a very powerful technique for characterizing carbohydrates. Depending upon the exact configuration of the MS detection system, it provides extra structural information on the eluted peaks, such as molar mass or fragmentation patterns. The technique is used both for the analysis of relatively simple mixtures of mono- and disaccharides and for more fundamental investigations into the structure of cell wall polysaccharides and hydrocolloids.
Ikegami et al. (14) described the use of a polyacrylamide modified monolithic silica capillary column for HILIC coupled with electron spray ionization (ESI)–MS detection for the analysis of carbohydrates. In their paper they presented chromatograms of mono- and disaccharide mixtures, of malto-oligosaccharides (ranging from DP1 to DP7), and di-saccharide mixtures (sucrose, maltose, and trehalose) in plant extracts.
Fu et al. (15) developed a general and efficient HILIC method for the separation of various kinds of carbohydrates, ranging from galacto-oligosaccharides, carrageenan oligosaccharides, sodium alginate, and chito-oligosaccharides, to higher molecular weight fructo-oligosaccharides.
In the laboratory of Food Chemistry of Wageningen University, HILIC–ELSD–MS is applied for the characterization of plant cell wall polysaccharides and exo-polysaccharides (16–18). The non-starch polysaccharides are subjected to a dedicated enzymatic hydrolysis using defined enzymes. The mixture of different oligosaccharides in the hydrolysate are then characterized and quantified with HILIC–ELSD–MS techniques, resulting in an enzymatic fingerprinting chromatogram. This approach provides information beyond the level of the monosaccharide composition of the polysaccharides. It also provides information on the distribution of the constituting monosaccharide moieties along the polysaccharide backbone or side-chains.
Sample Preparation
As a final note, two remarks on sample preparation and sample cleanup should be noted. Firstly, the degree of sample pretreatment depends very much on which chromatographic detector is applied for the analysis. Applying high selective carbohydrate detectors such as PAD and MS, generally requires a less intensive sample cleanup procedure than when applying an universal RI detector.
Secondly, most sugars and oligosaccharides are water-soluble. Therefore it is common practice to apply an aqueous extraction of the sugars and oligosaccharides as a first step in the analytical procedure. However, depending on the sample origin, it is possible that the sample contains some (rest) enzyme activities (19) as amylases or amyloglucosidases. In that case, measures should be taken to ensure that the carbohydrate composition in the sample is not changed as a result of enzymatic activities during the sample cleanup procedure.
Kommer Brunt is from the Carbohydrate Competence Centre, Eurofins Food Testing Netherlands in Heerenveen, The Netherlands. He is currently with Rotating Disc BV, Haren, The Netherlands. Please direct correspondence to: kommer.brunt@planet.nl
References
(1) Commission directive 2008/100/EU Annex II, October 28, 2008.
(2) Report on the 30th session of the Codex Committee on nutrition and foods for special dietary uses, Capetown, South Africa, November 3–7, 2008, ALINORM 09/32/26.
(3) FAO Technical meeting report on prebiotics, September 15–16, 2007.
(4) K. Brunt, J. Chromatogr. 246, 145–151 (1982).
(5) K. Brunt, J. Chromatogr. 267, 347– 354 (1983).
(6) S. Hughes and D.C. Johnson, J. Agric. Food Chem. 30, 712–714 (1982).
(7) S. Hughes and D.C. Johnson, Anal. Chim. Acta 149, 1–10 (1983).
(8) T.R.I. Cataldi and C.C.G.E. De Benedetto, Fresenius J. Anal. Chem 368, 739–758 (2000).
(9) C. Corradini, A. Cavazza, and C. Bignardi, Int. J. Carbohydr. Chem. 13, (2012).
(10) ISO 11292, Instant coffee — Determination of free and total carbohydrate contents — method using high performance anion-exchange chromatography. February 1995.
(11) CEN/TS 15754, Animal feeding stuffs — Determination of sugar content — high performance anion-exchange chromatographic method (HPAEC–PAD), January 2008.
(12) A.J. Alpert, J. Chromatogr. 499, 177–196 (1990).
(13) G. Karlsson, S. Winge, and H. Sandberg, J. Chromatrogr. A 1092, 246–249 (2005).
(14) T. Ikegami, K. Horie, N. Saad, K. Hosoya, O. Fiehn, and N. Tanaka, Anal. Bioanal. Chem. 391, 2533–2542 (2008).
(15) Q. Fu, T. Liang, X. Zhang, Y. Du, Z. Guo, and X. Liang, Carbohydr. Res. 345(18), 2690–2697 (2010).
(16) A.M.G. Leijdekkers, M.G. Sanders, H.A. Schols, and H. Gruppen, J. Chromatogr. A 1218, 9227–9235 (2011).
(17) C. Remoroza, S. Cord-Landwehr, A.G.M. Leijdekkers, B.M. Moerschbacher, H.A. Schols, and H. Gruppen, Carbohydr. Polymers 90, 41–48 (2012).
(18) M.M. Kool, H. Gruppen, G. Sworn, and H.A. Schols, Carbohydr. Polymers 98, 914–921 (2013).
(19) M. Karkacier, M. Erbas, M.K. Ulsu, and M. Aksu, J. Chromatogr. Sci. 41, 331–333 (2003).

Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.









