Advances in Micro-Ultrahigh-Performance Liquid Chromatography–Mass Spectrometry in Residue and Contaminant Analysis
Special Issues
Advances in nano-ultrahigh-performance liquid chromatography (nUHPLC) and "plug-and-play" micro-UHPLC (?UHPLC) for the detection of veterinary drugs and steroids in porcine meat and urine respectively are described.
Advances in nano-ultrahigh-performance liquid chromatography (nUHPLC) and "plug-and-play" micro-UHPLC (µUHPLC) for the detection of veterinary drugs and steroids in porcine meat and urine respectively are described. Recent developments in "plug-and-play" µUHPLC devices offer several advantages compared to earlier micro-LC systems. As well as the ease of use, solvent consumption can be reduced by more than 95% and the amount of sample required can be reduced 10-fold. In addition, the performance and the robustness of the µUHPLC sytem described is comparable to conventional UHPLC, and can be successfuly applied for the routine analysis of residues and contaminants in food.
Residues and contaminants that can affect consumer safety (such as veterinary drugs, growth promoters, pesticides, or natural toxins) are continually monitored to ensure product safety and compliance with legislation (1). Residues and contaminants may be present at low concentrations in the ng/kg to µg/kg range, and so most laboratories use liquid chromatography coupled to tandem mass spectrometer (LC–MS–MS). Significant improvements have been made over the last decade, with LC methods shifting from traditional particles (3–5 µm) to ultrahigh-performance liquid chromatography (UHPLC) using smaller particles (1.7–2-µm). This has led to narrower peaks, which has improved peak capacity and detectability. Improvements in MS instruments have increased sensitivity and acquisition speeds (required for quantitative measurement of the narrow peaks). Furthermore, MS instruments have become compatible with the higher flow rates typically used in UHPLC. Many laboratories have transferred existing HPLC methods to UHPLC in combination with highly sensitive and fast scanning MS–MS instruments.
The currently applied UHPLC techniques in the field of routine food analysis can be divided in different classes: low flow UHPLC (flow rate = 10–100 µL/min); conventional UHPLC (100–600 µL/min); and high flow UHPLC (>600 µL/min). Nano- or micro-liquid chromatography [nLC (<1 µL/min) or µLC (1–10 µL/min)] are missing from this list because they have not been routinely used in the field of routine food safety analysis for a number of reasons.
Variable flow rates were one major issue with nLC and µLC attributed to nano- or micro-flows that were attained by splitting regular flow rates using capillaries; the viscosity of the mobile phase changes during analysis; and injection of dirty samples that could cause clogging. This hampered the implementation in routine laboratories because unstable flow rates caused unstable retention times that meant the methods could not be used for official control purposes where it is mandatory that retention times are consistent to allow proper identification (2,3). This was especially true in laboratories where a technique is performed on a daily and routine basis.
Another drawback of early nLC or µLC techniques were issues associated with the connections between the autosampler, trapping columns, analytical columns, and electrospray emitters. Each connection would introduce void volumes that eventually resulted in serious peak broadening. Moreover, detecting a small leakage with all these connections was a challenging task at low nano or micro flow rates. This was almost impossible for routine applications where various applications would be performed on a single instrument and columns would have to be frequently changed. For these reasons outlined, conventional nLC and µLC were not really applicable for routine applications (4).
Nowadays, there are several commercial nLC and µLC instruments available, without the flow rate issues discussed above, that are able to produce stable nano- or micro-flow without using flow splitting. The most recent nano pumps can produce stable low flow rates of 10–4000 nL/min and handle a pressure of 10,000 psi (approximately 700 bar). This high-pressure tolerance made it possible to use nano or microbore columns packed with sub-2-µm particles. nUHPLC and µUHPLC are now becoming the standard in nano and microLC separations.
A major issue remaining is the connection of pre-columns and analytical columns to both the nUHPLC and nano electrospray emitter, restricting the implementation of the technique in the field of routine food control. In response to this, n(U)HPLC and µ(U)HPLC devices have been developed that can be directly connected to the electrospray source or LC instrument without manual connections. These solutions lead to more robust equipment as no manual connections have to be made. Potential advantages of the nLC and µLC are reduced use of high grade solvents and reference standards. Furthermore, at these lower flow rates in theory the detectability is improved and ionization suppression is reduced (5–7). To explore the applicability of nUHPLC and µUHPLC in food safety, the levels of veterinary drugs and steroids in porcine meat and urine were determined.
Experimental Veterinary Drug Screening
Reagents
Water for sample preparation was deionized and passed through a Milli-Q water purification system (Millipore). Formic acid (98–100%) was purchased from Merck. Leucine-enkephalin was purchased from Sigma-Aldrich. Acetonitrile (HPLC supra gradient), methanol (absolute) and water (UHPLC–MS-grade) were purchased from Biosolve. Veterinary drug analytical standards were purchased from Sigma-Aldrich, Smith Kline Beecham, Riedel de Haen, or Fluka. Oxfenbendazole and oxfenbendazole sulfon were purchased from Syntex, valnemulin from Novartis, and marbofloxacin from Vetoquinol. Albendazole (sulfoxide), oxfendazole (sulfone), hydroxyl-ipronidazol, carazolol, piroxycam, propyphenazone, and piroxicam were obtained from Bundesamt fur Verbraucherschutz und lebensmittelsicherheit (BVL–EURL).
Sample Preparation
Blank porcine samples were selected from the Dutch national monitoring program. These samples (n=7) were fortified with the various veterinary drugs to develop the screening method. Extraction was done as previously described by Peters et al. (8), and µSPE was subsequently performed. Of the acetonitrile/water extract, 375 µL was diluted with water to a volume of 3.75 mL. A 30-µm 96-well SPE Oasis HLB µElution plate (Waters) was conditioned and equilibrated with 200 µL methanol and water, respectively, and 2850 µL of diluted extract was applied to the plate. The cartridge was washed with 500 µL water/methanol (95/5, v/v), and the veterinary drugs then eluted into a 96-well plate using 500 µL acetonitrile/methanol (50/50, v/v). The eluate was evaporated to dryness at 40 °C under a stream of nitrogen. The residue was reconstituted in 50 µL water/acetonitrile (90/10) containing 0.1% (v/v ) formic acid. The well plate was sealed using a pealable heat seal to prevent evaporation.
UHPLC Separations
UHPLC was performed on an Acquity UPLC system (Waters) equipped with a 100 mm × 2.1 mm, 1.7-µm dpUPLC BEH C18 column. Mobile phase A and B were water and acetonitrile, respectively, that both contained 0.1% (v/v) formic acid. Gradient flow rate was 400 µL/min starting at 0% B, which was increased linearly to 40% B in 4 min and then linearly increased in 6 min to 100% B. It was kept at 100% B for 2 min and subsequently returned to 0% B. An equilibration time of 4 min was allowed prior to the next injection. The column temperature was kept at 40 °C and 20 µL was injected onto the column.
µUHPLC Conditions
For µUHPLC a packed micro LC column (50 mm × 0.15 mm, 1.7-µm dp BEH C18 [Waters]) was used. The microbore column, microfluidics connections, and ESI emitter were integrated on a ceramic tile (iKey, Waters) that was inserted into a dedicated source module (ionKey/MS, Waters) coupled to the MS. Solvent and sample were delivered to the ionKey/MS module using a Waters nanoAcquity UPLC binary solvent manager and nanoAcquity sample manager, respectively. The gradient was started at 1% B which then linearly increased to 50% B in 10 min and then increased to 95% B in 0.1 min. This composition was held for 2 min and then the composition was changed back to 1% B in 1 min. An equilibration time of 1 min was allowed prior to the next injection. The flow rate was kept constant at 4 µL/min. The column temperature was kept at 45 °C and 2 µL of each sample was injected.
nUHPLC Conditions
For nUHPLC, a nano-Acquity UPLC system (Waters) was equipped with a 20 mm × 0.2 mm, 5-µm dpHSS T3 trap column (Waters) and a 100 mm × 0.1 mm, 1.7µm dp BEH C18 analytical column (Waters). The mobile phase composition was the same as described in the UHPLC section. The compounds of interest were trapped for 2 min with a flow of 7.5 µL/min. After trapping, a gradient was run at a flow rate of 0.6 µL/min starting at 1% B, which was increased linearly to 50% B in 11 min and then linearly increased in 5 min to 95% B. It was kept at 95% B for 5 min and subsequently returned to 1% B in 3 min. An equilibration time of 5 min was allowed prior to the next injection. The column temperature was kept at 35 °C and 2 µL was injected onto the column.
Mass Spectrometric Detection
The UHPLC and nUHPLC were directly interfaced with a Xevo Q-ToF mass spectrometer (Waters) equipped with a electrospray ionization interface (ESI) and nano ESI, respectively. For µUHPLC the column and electrospray emitter were integrated in one module, which was inserted into a dedicated source mounted onto the Q-ToF MS as already described above. The analysis was performed in ESI positive (ESI+) mode. A capillary voltage of 3 kV and a cone voltage of 40 V were applied.
Steroid Drug Screening
Reagents
17β-boldenone (17β-Bol), 1,4-androstadiene-3,17-dione (ADD), methylboldenone (meBol), norethandrolone (norEth), 17α-methyltestosterone (17α-meT), 17α-nortestosterone (17α-norT), 17β-nortestosterone (17β-norT), 17α-testosterone (17α-T), 17β-testosterone (17β-T), and 4-chlorotestosterone (clostebol, clos) were obtained from Steraloids. 17α-boldenone (17α-Bol), 17α-trenbolone (17α-Tren), 17β -trenbolone (17β-Tren), 4-chloro-4-androst-3,17-dione (CLAD), 17β-testosterone-d3 (17β-T-d3), 17β-boldenone-d3 (17β-Bol-d3), 17β-trenbolone-d3 (17β-tren-d3), methylboldenone-d3 (meBol-d3), 17β-nortestosterone-d3 (17β-norT-d3), and 17α-methyltestosterone-d3 (17α-meT-d3) were obtained from the European Reference Laboratory. 16βOH-stanozolol (16βOH-Stan), stanozolol (Stan) and stanozolol-d3 (Stan-d3) were purchased from Cerilliant and tetrahydrogestrinone (THG) was purchased from the National Measurement Institute in Pymble, Australia.
Sample Preparation
Porcine urine samples were screened for the presence of steroids as a second example matrix. Samples were treated using β-glucuronidase and arylsulphatase to deconjugate the excretion products to their aglycon/desulphonated form. The samples were then applied to a dual stage SPE cleanup using a C18 (Varian bond Elut) and subsequently a NH2 cartridge (IST Isolute, Biotage). After evaporation, the cleaned extract was further purified using HPLC fractionation. The purified extracts were evaporated to dryness and reconstituted in 225 µL water/methanol (70/30, v/v) containing 0.3% formic acid.
UHPLC Conditions
Instrument set-up was as previously described. The gradient started at 10% B which was linearly increased to 20% B in 2 min, and 20% to 60% B in 6 min. The gradient was then increased in 0.1 min to 95% B which was kept for 2.9 min before returning to 10% B in 0.5 min. An equilibration time of 3.5 min was allowed before the next injection. The flow rate was kept constant at 392 µL/min, the column temperature at 40 °C, and the injection volume was 5 µL.
µUHPLC Conditions
The steroids were separated using a 100 mm × 0.15 mm, 1.7-µm dp BEH C18 column, applying a flow rate of 2 µL/min. The gradient conditions were kept identical as the conditions described in the UHPLC part. The column temperature was kept at 40 °C and an injection volume of 0.5 µL was used.
Mass spectrometric detection
The UHPLC–ESI and µUHPLC were connected to a Xevo TQ-S triple quadrupole MS (MS–MS) [Waters] in the same way as described for the veterinary drugs. The mass spectrometer was operated in ESI+, with a capillary voltage of 3.2 kV. Argon was used as collision gas at a flow of 0.15 mL/min. The cone voltage and collision energies were optimized for the various steroids by direct infusion experiments. For each of the steroids two SRM transitions were monitored.
Results and Discussion
Comparison of UHPLC, nUHPLC, and µUHPLC in Veterinary Drug Screening
Porcine meat samples were screened for the presence of veterinary drugs using UHPLC, nUHPLC, and µUHPLC. In principle, when scaling down UHPLC methods to nUHPLC, the injection volume has to be reduced proportionally with column diameter and flow rate to avoid band broadening, achieve adequate peak shapes, and maximize sensitivity. Factoring in the column and flow rate, this translates into an injection volume of 45 nL (compared to the 20 µL used in UHPLC). Even with the theoretical sensitivity gain, this results in an unacceptable increase in the limit of detection. To compensate for this, a relatively large volume of extract (2 µL) was injected adding 5 min to the analysis time. It also negatively affects peak shape because of insufficient refocusing on the head of the analytical column. To remove this issue, a precolumn was used to transfer the extract at a higher flow rate, the analytes were trapped and subsequently transferred as a narrower band to the analytical column.
The use of a precolumn is not ideal when developing multi-compound methods as a wide variety of polarities and functional groups are present. The first trapping columns available for the nUHPLC system contained C18 particles, but when used for screening for veterinary drugs it caused peak broadening for some of the compounds (Figure 1). For example, the peak width of sulfadoxine (peak 2) and sulfadimethoxine (peak 3) was relatively broad. To improve peak shape a different trap material containing high strength silica C18 particles was used, which significantly improved peak shape, especially for sulfadoxine (peak 2) and sulfadimethoxine (peak 3). Although this high strength silica C18 column is more retentive a small shift in retention could be observed which is potentially attributable to the improved end-capping of the silica in this trap column. This improvement reduced the secondary interactions of sulfone and sulfonic acid groups under acidic mobile phase with free silanol groups at the silica surface.
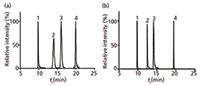
Figure 1: nUHPLCâMS chromatograms obtained using different trapping columns for peak refocusing: (a) C18; (b) high strength silica C18. Analytes: 1) 5-OH thiabendazole, 2) sulfadoxine, 3) sulfamethoxine, and 4) mefenemic acid.
To elute the compounds as a sharp plug, the trap column has to be maintained at an optimal temperature, but with the nUHPLC system used this was not possible. With a modified setup it was possible to place both trap and analytical column at optimal temperatures. Unfortunately, under the modified setup, the capillaries were under a lot of stress, which affected the robustness of the system.
The developed nUHPLC method was compared with the UHPLC method in terms of sensitivity for a number of compounds (Table I). On average the signal-to-noise (S/N) improved with a factor 5 and the areas of the compounds with a factor 18. Factoring in differences in injection volume between UHPLC (20 µL) and nUHPLC (2 µL) the improvement in detectability (S/N) is 50-fold. A major drawback of the nUHPLC was that the run-time increased from 16 min with UHPLC to 33 min with nUHPLC. This, in combination with the inflexibility of rapidly changing columns for different applications, hampers the implementation in routine. Plug-and-play µUHPLC offers an alternative method.

Table I: Improved signal-to-noise ratio: Detectability in nUHPLC and conventional UHPLC
µUHPLC was performed with a flow rate of 4 µL/min, and no trapping was performed. The loop of 2 µL was flushed at a flow rate of only 4 µL/min. Therefore, injection volume and solvent are of major importance for a good separation and peak shape. A small percentage organic present in the injection solvent may already cause poor peak shapes and loss of retention. For the early eluting veterinary drugs a small injection volume will cause in-loop dilution that results in broader peaks.

Table II: Differences between UHPLC, µUHPLC, and nUHPLC applications for veterinary drugs
The developed µUHPLC method was validated according to EU criteria (2). Preliminary results were promising for example: Carazolol at levels of 5, 10, and 15 µg/kg in porcine meat had good linearity (correlation 0.991), accuracy (89–95%), repeatability (11–15%) and within-laboratory reproducibility (12–16%). The robustness and performance of the µUHPLC system is comparable with that of UHPLC.
Comparison of UHPLC and nUHPLC in Steroid Screening
For the steroid application UHPLC–MS-MS and µUHPLC–MS-MS were compared. Steroids of interest eluted at a higher organic solvent concentration and therefore were better focused at the head of the analytical column. When transferring the method from UHPLC to µUHPLC, column volumes and gradient were comparable by scaling the flow rate of the UHPLC method to the µUHPLC method. Based on the internal diameter of the column, the flow rate was adjusted, respectively to 392 µL/min in the UHPLC column and 2 µL/min in the µUHPLC column. Also the injection volume was reduced (from 5 µL with the conventional UHPLC and 0.5 µL with µUHPLC) but not proportionally to achieve at least the same limits of detection. Figure 2a shows that with a 10-fold smaller injection volume, the sensitivity of the steroids analyzed was improved. The repeatability of the method was also comparable to the UHPLC method.
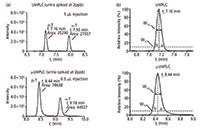
Figure 2: (a) Sensitivity of α-testosterone and β-testosterone. (b) Difference in peak shapes in UHPLC and µUHPLC.
The introduction of less matrix into the system reduced drift in sensitivity during analysis. Retention times observed for both the UHPLC and µUHPLC were extremely stable, with deviations smaller than 5 s within each series. When studying the chromatographic peaks in more detail, as shown in Figure 2b, the asymmetry factor (a/b) was only slightly affected. On average the steroids in UHPLC had an asymmetry factor of 1.37±0.34 versus 1.39±0.11 in µUHPLC. The effective plate numbers of both columns were in the same range, UHPLC Neff = 48711 and µUHPLC Neff = 46829. It could be concluded that the plug-and-play µUHPLC method is at least delivering the same performance quality as conventional UHPLC.
Green Chemistry
Downscaling analytical procedures to reduce solvent and plastic waste is key to sustainable chemistry. Here, all methods developed were miniaturized to reduce waste. SPE was downscaled from the original method using conventional SPE cartridges (60 mg/3 mL) to a 96-well microplate. Conventional SPE used a total of 12 mL of solvent, whereas µSPE used only 1.4 mL of solvent, a reduction of almost 90%. The UHPLC method used 6.4 mL of mobile phase, compared to µUHPLC that used only 60 µL, a 99.9% reduction. Furthermore, the sample size (injection volume) can also be reduced by up to a factor of 10 when using µUHPLC. The use of smaller injection volumes provide new opportunities in food safety analysis, such as combining this technique with bio-activity driven sample cleanup (10,11).
Conclusions
In conclusion, µUHPLC as a technique is at the same level as conventional UHPLC and the robustness of the technique means that it can be implemented in routine laboratories. Furthermore, the green analytical concept is of interest from both an economic and sustainability perspective. We expect that the type of "plug-and-play" µUHPLC techniques used here will be adapted in different applications, such as food safety analysis, and more "plug-and-play" µUHPLC equipment will becomes more commercially available on the market.
Arjen Gerssen is a junior scientist in the group of natural toxins and pesticides. His main expertise is in the field of phycotoxins. He also has a strong interest in applying new separation and MS technologies for residue and contaminant analysis.
Marco Blokland is a junior scientist involved in the European Union Reference Laboratory for growth promoting compounds. The research topics covered include the whole range of growth promoters, from small molecules and peptides to proteins. Currently he is working on the use of statistical models to detect natural hormone abuse in cattle.
Hans G.J. Mol is a senior scientist and heads the group of National Toxins and Pesticides at RIKILT. He has over 15 years experience in residue and contaminant analysis in the food chain using chromatography with a range of mass spectrometric techniques. Please direct correspondence to: Hans.Mol@wur.nl
References
(1) European Commission (1996), 96/23/EC, Off J Eur Commun L125:10–32.
(2) European Commission (2002), 657/2002/EC, Off J Eur Commun L221:8–36
(3) European Commission(2013_ SANCO/12571/2013. Available at: http://www.eurl-pesticides.eu/library/docs/allcrl/AqcGuidance_Sanco_2013_12571.pdf
(4) C.M. Harris, Anal. Chem. 75(64a–69a), (2003).
(5) J.P. Chervet, M. Ursem, and J.B. Salzmann, Anal. Chem. 68, 1507–1512 (1996).
(6) A. Kloepfer, J.B. Quintana, and T. Reemtsma, J. Chromatogr. A 1067, 153–160 (2005).
(7) L. Rieux, E.J. Sneekes, R. Swart, and M. Swartz, LCGC North Amer. 29(10), 926 (2011).
(8) R.J. Peters, Y.J. Bolck, P. Rutgers, A.A. Stolker, and M.W. Nielen, J. Chromatogr. A 1216, 8206–8216 (2009).
(9) S. Armenta, S. Garrigues, and M. de la Guardia, TrAC 27, 497–511 (2008).
(10) P. Aqai, J. Peters, A. Gerssen, W. Haasnoot, and M.W.F. Nielen, Anal. and Bioanal.Chem. 400, 3085–3096 (2011).
(11) P. Aqai, E. Cevik, A. Gerssen, W. Haasnoot, and M.W.F. Nielen, Anal. Chem. 85, 3255–3262 (2013).

Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.









