The 2024 Lifetime Achievement and Emerging Leader in Chromatography Awards
Wolfgang F. Lindner and Martina Catani are the winners of the 17th annual LCGC Lifetime Achievement and Emerging Leader in Chromatography Awards, respectively. These awards honor the work of talented separation scientists at different stages in their career. The award winners will be honored in March in an oral symposium at the Pittcon 2024 conference in San Diego, California.
The Lifetime Achievement Award
Wolfgang Lindner is the 2024 Lifetime Achievement in Chromatography Award recipient. The award honors an outstanding and seasoned professional for a lifetime of contributions to the advancement of chromatographic techniques and applications (Table I).

Wolfgang F. Lindner, the 2024 winner, is chair of Analytical Chemistry at the University of Vienna. Lindner is a prominent figure in the realm of chiral separations, particularly in analytical and preparative liquid chromatography (LC), as well as in contemporary separation methods such as capillary electrophoresis (CE), capillary electrochromatography (CEC), and supercritical fluid chromatography (SFC). Rather than solely focusing on plate counts, Lindner’s expertise lies in enantioselective molecular recognition, especially concerning polar and ionic species. His notable contributions encompass the development and licensing of various chiral stationary phases, which have found widespread use among researchers and within the pharmaceutical industry. His work extends to the application of enantiomer separations in the fields of chiral drug pharmacokinetics and the metabolomics of endogenous compounds, including amino and hydroxyl acids. “I am known best for the field of enantioselective chromatography,” Lindner said.

At the core of Lindner’s research is a deep understanding of non-covalent binding interactions between analytes and chiral stationary phases, as well as the roles of accompanying mobile phase components. His innovations expand into the realm of highly selective mixed-mode and hydrophilic interaction chromatography (HILIC) phases, along with arginine modifications tailored for liquid chromatography–mass spectrometry (LC–MS) analysis of highly basic peptides. Throughout his work, Lindner remains committed to fundamental physico-chemical principles, striving to create exceptionally selective separations.
IMAGE 1: Lindner at the Second International Symposium on Chiral Discrimination (ISCD) in Rome (1991) (All images provided by Wolfgang Lindner).

As a teenager, Lindner became fascinated by chemistry. “Curiosity drove me my whole life and still does it today,” he said. Beginning his academic research journey in the late 1970s, Lindner entered a landscape where chiral separations were an art, but his efforts, combined with those of other leaders, have transformed it into a routine practice.
“I became specifically fascinated by chirality as a property and shape descriptor of molecules and thus by the concepts to enable resolution of chiral molecules with chromatographic, electrophoretic, and crystallization technologies,” Lindner said.
Notably, he introduced O,O-diacyltartaric acid anhydrides as chiral derivatizing agents in the mid-1980s, a contribution that continues to impact the analysis and production of enantiomerically pure drugs. In the mid-1990s, he introduced cinchona-based chiral anion exchange columns, followed by a chiral cation exchange column. However, his most significant breakthrough involved the development of chiral zwitterionic stationary phases capable of separating positive, negative, and ampholytic compounds. This ingenious approach combined a chiral amidosulfonic acid with quinine and quinidine scaffolds. Many of these columns have been licensed and commercialized, significantly influencing the pharmaceutical industry. Lindner’s enduring legacy is evident in the transformation of chiral separations from an art to a routine science.
“The success of modern HPLC is driven by its power of selectivity (multidimensional), efficiency, speed, scalability (nano to process), productivity, hyphenation (with mass spectrometry), and applicability (even for complex samples),” he said.
IMAGE 2: HPLC 2014 in New Orleans, USA (left to right): Prof. Frantisec Svec, Prof. Massimo Morbidelli, and Wolfgang Lindner.

Main Research Topics
Lindner’s major research interests have focused on the power of selectivity in LC.
“My research interests were always driven by the elucidation of the fundamentals of the interactions of molecules to each other,” he said. Lindner’s passion has culminated in the development of diverse and useful methodological concepts for LC systems. The areas of particular interest to Lindner include:
- Reversed-phase separation of optical isomers using chiral metal chelate additives
- Strong detrimental effect of enantiomeric impurities on enantioselectivity
- Liquid chromatographic separation of enantiomeric alkanolamines
- Quinine and quinidine derivatives as chiral selectors
- Elucidation of chiral recognition mechanisms of cinchona alkaloid carbamate receptors
- Chiral recognition of peptide enantiomers by cinchona alkaloid derived chiral selectors
- Direct high-performance liquid chromatographic separation of peptide enantiomers
- Novel strong cation exchanger type chiral stationary phase
- Synergistic effects on enantioselectivity of novel zwitterionic chiral stationary phases
- Chiral monolithic columns for enantioselective capillary electrochromatography.
IMAGE 3: Chirality 2017 Tokyo in Japan (left to right at the drum): Prof. Yoshio Okamoto, Prof. Wolfgang Lindner, and Prof. Nina Berova.

Major Research Contributions
Lindner’s transformative journey in separation science has been characterized by groundbreaking contributions that have revolutionized the field. As a postdoctoral researcher in the late 1970s, at Barry Karger’s lab in Boston, Lindner’s work introduced a pioneering concept of chiral analyte separation using chiral selectors in the mobile phase via chiral ligand exchange chromatography (CLEC) (1). This experience set the stage for his remarkable career in chiral chromatography, where he skillfully harnessed his strong organic chemistry background to design novel chiral systems with exceptional selectivity, achieving impressive alpha values as high as 100 (2).
Together with luminaries such as Pirkle, Armstrong, and Okamoto, Lindner is revered as an expert in the liquid chromatographic separation of enantiomers. Their collective efforts catalyzed the widespread adoption of analytical and preparative chiral columns, playing a pivotal role in drug synthesis and chemical research. The significance of this impact is exemplified by the 2021 Nobel Prize in Chemistry, acknowledging the role of chiral chromatography in enabling advancements like asymmetric catalysis. This prize was awarded to Benjamin List and David W. C. MacMillan “for the development of asymmetric organocatalysis.” Major pharmaceutical companies have been empowered by efficient facilities for racemic drug separation on a preparative scale and the screening of individual enantiomers to decipher their pharmacological profiles. Continuing his trajectory of direct enantiomer separation, Lindner introduced diacyl tartaric acid anhydride as a chiral derivatizing agent in the mid-1980s, enabling excellent separation of diastereomeric species (3). This patented approach, even three decades later, continues to impact drug production, as seen with (S)-Timolol.HCl, a potent antiglaucoma medication.
But Lindner’s most enduring legacy resides in his transformative contributions to chiral stationary phases, particularly ion exchangers. His pursuit of chargeable chiral selector species led him to the fundamental alkaloids, quinine and quinidine, in the 1990s. These pseudo-enantiomeric diastereoisomers, characterized by a chiral tertiary amino group within the quinuclidine moiety, possess remarkable stereochemical capabilities. Lindner’s meticulous studies, both spectroscopic and chromatographic, culminated in the addition of a carbamate group near the chiral centers, facilitating highly specific hydrogen bonding that significantly enhanced chiral recognition (4–7).
The grafting of the chiral selector onto porous silica packing led to the pioneering enantioselective anion exchanger, which has now been commercially available for more than 20 years (4). His subsequent innovation in 2008, incorporating a negatively charged chiral sulfohexoylcarbamate scaffold into a positively charged quinine or quinidine selector motif, created the widely recognized zwitterionic chiral stationary phase (8), permitting comprehensive chiral separations of anions, cations, and ampholytes, with applications spanning amino acids and peptides (9).
Lindner’s far-reaching contributions extend beyond liquid chromatography into capillary electrophoresis (10), capillary electrochromatography (11), supercritical fluid chromatography (12), and affinity chromatography (13,14). His focus on leveraging chemistry for selectivity has expanded stereoselectivity beyond enantioselectivity, with his ion-exchange columns serving as effective mixed-mode (15) and hydrophilic interaction chromatography (16) stationary phases.
IMAGE 4: Discussion of molecular interaction phenomena between Prof. Stellan Hjertie and Wolfgang Lindner, at 17th International Symposium on Microscale Separations and Capillary Electrophoresis (HPCE 2004) in Salzburg, Austria.

Key Awards and Honors
Lindner believes that when working in research-focused environments, scientists must accept a collegial-like competition among colleagues on scientific achievements.
“It affords a constant engagement with the latest developments in the field,” he said. Lindner himself has been selected by many colleagues for various scientific awards. Below, you can find a list of all the major awards he has won.
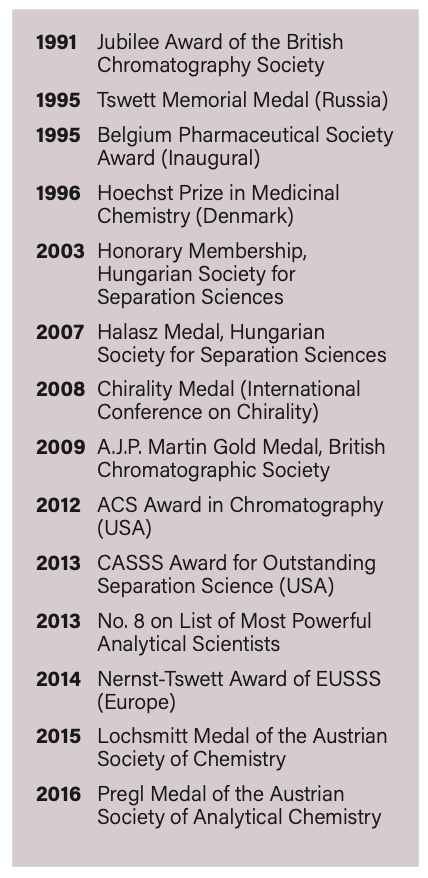
Service to the Scientific Community
Lindner’s contributions are substantial and multifaceted. His academic impact is evident through a prolific publication record, encompassing 590 peer-reviewed papers, with an h-index of 71 and over 22,100 citations. Notably, he holds 17 patents, several of which have been licensed for commercial products. His influence also extends to education, having mentored over 100 Master’s and PhD students, as well as more than 15 extended-stay postdoctoral researchers, many of whom have achieved distinguished careers in separation science globally. Examples of his notable mentees include Michael Lammerhofer, Kevin Schug, Alexander Leitner, and others. Lindner has found that individual and personalized mentoring of coworkers and students is a particularly challenging and a rewarding task.
Lindner’s prominence is demonstrated by his extensive involvement in scientific conferences. He has delivered over 100 plenary, keynote, and research lectures at major symposia, in addition to participating in more than 100 named lectureships and lectures at universities and industries. His support for young scientists is evident through his mentorship and more than 100 posters presented by students and collaborators at significant symposia. Furthermore, his role as chairman in various scientific meetings, such as the International Symposium on Liquid Chromatography and the International Symposium on Chromatography, showcases his dedication to advancing the field.
His contributions to scientific journals are substantial; he served as editor for the Journal of Chromatography B from July 1995 to June 2006, and he remains active on several Editorial Advisory Boards for esteemed journals including LCGC, Chirality, Chromatographia, Journal of Chromatography B,Journal of Pharmaceutical and Biomedical Analysis, International Journal of Bio-Chromatography, and Journal of Analytical and Bioanalytical Chemistry.
Lindner’s commitment to nurturing the next generation of analytical scientists is palpable through his establishment of the Austrian Summer Training in LC–MS and the Austrian Young Analysts Forum, providing platforms for skill development and networking. His leadership within science societies is also evident, having served as President of the Austrian Society of Analytical Chemistry and currently serving as its Honorary President.
IMAGE 5: The Lindner working group in 2004 and 2005, Seminar Room of the Institute of Analytical Chemistry of the University of Vienna. Highlighted is Michael Lämmerhofer, he was for 20 years a co-worker of Wolfgang Lindner. He joined his working group in 1991 at the University of Graz (AT) and moved with him 1991 to the University of Vienna. The picture of the working group was taken in 2009 in the Seminar Room of the Institute of Analytical Chemistry of the University of Vienna.

In addition to his prolific publication record, Lindner’s advances have been widely commercialized and adopted, significantly transforming chiral separations from an art to a routine procedure. His mentorship has paved the way for students who have embarked on major careers in separation science, while his leadership in international meetings and committee service underscores his commitment to advancing the separation field (17). As an indisputable candidate for the LCGC Lifetime Achievement Award, Wolfgang Lindner’s exceptional impact resonates throughout the chromatographic separation science arena, shaping its trajectory with innovation and excellence.
References
(1) Lindner, W.; LePage, J. N.; Davies, G.; Seitz, D. E.; Karger, B. L. Reversed Phase Separation of Optical Isomers of Dns-Amino Acids and Peptides Using Chiral Metal Chelate Additives. J. Chromatogr. A 1979, 185, 323–343. DOI: 10.1016/S0021-9673(00)85612-5
(2) Levkin, P. A.; Maier, N. M.; Schurig, V.; Lindner, W. Strong Detrimental Effect of a Minute Enantiomeric Impurity of a Chiral Selector on the Enantioselectivity Factor. Angew. Chem. Int. Ed., 49 (42), 7742–7744. DOI: 10.1002/anie.201002215
(3) Lindner, W.; Leitner, C.; Uray, G. Liquid Chromatographic Separation of Enantiomeric Alkanolamines via Diastereomeric Tartaric Acid Monoester. J. Chromatogr. A 1984, 316, 605–616. DOI: 10.1016/S0021-9673(00)96186-7
(4) Lämmerhofer, M.; Lindner, W. Quinine and Quinidine Derivatives as Chiral Selectors. I. Brush Type Chiral Stationary Phases for HPLC Based on Cinchonan Carbamates and Their Application as Chiral Anion Exchangers. J. Chromatogr. A1996, 741, 33–48. DOI: 10.1016/0021-9673(96)00137-9
(5) Maier, N. M.; Schefzick, S.; Lombardo, G. M.; Feliz, M.; Rissanen, K.; Lindner, W.; Lipkowitz, K. B. Elucidation of the Chiral Recognition Mechanism of Cinchona Alkaloid Carbamate-type Receptors for 3,5-Dinitrobenzoyl Amino Acids. J. Am. Chem. Soc.2002, 124 (29), 8611–8629. DOI: 10.1021/ja020203i
(6) Czerwenka, C.; Zhan, M. M.; Kählig, H. P.; Maier, N. M.; Lipkowitz, K. B.; Lindner, W. Chiral Recognition of Peptide Enantiomers by Cinchona Alkaloid Derived Chiral Selectors: Mechanistic Investigations by Liquid Chromatography, NMR Spectroscopy and Molecular Modeling. J. Org. Chem. 2003, 68 (22), 8315–8327. DOI: 10.1021/jo0346914
(7) Czerwenka, C.; Lämmerhofer, M.; Maier, N. M.; Rissanen, K.; Lindner, W. Direct High Performance Liquid Chromatographic Separation of Peptide Enantiomers: Study on Chiral Recognition by Systematic Evaluation of the Influence of Structural Features of the Chiral Selectors on Enantioselectivity. Anal. Chem. 2002, 74 (21), 5658–5666. DOI: 10.1021/ac020372l
(8) Hoffmann, C.; Lämmerhofer, M.; Lindner, W. Novel Strong Cation Exchanger Type Chiral Stationary Phase for the Enantiomeric Separation of Chiral Amines by High-Performance Liquid Chromatography. J. Chromatogr. A2007, 1161 (1-2), 242–251. DOI: 10.1016/j.chroma.2007.05.092
(9) Hoffmann, C.; Pell, R.; Lämmerhofer, M.; Lindner, W. Synergistic Effects on Enantioselectivity of Novel Zwitterionic Chiral Stationary Phases for Separations of Chiral Acids, Bases, and Amino Acids by HPLC. Anal. Chem. 2008, 80 (22), 8780-8789. DOI: 10.1021/ac801384f
(10) Lämmerhofer, M.; Zarbl, E.; Lindner, W. tert.-Butylcarbamoylqunine as Chiral Ion-pair Agent in Non-aqueous Enantioselective Capillary Electrophoresis Applying the Partial Filling Technique. J. Chromatogr. A 2000, 892 (1-2), 509–521. DOI: 10.1016/S0021-9673(00)00172-2
(11) Lämmerhofer, M.; Peters, E. C.; Yu, C.; Svec, F.; Fréchet, J. M. J.; Lindner, W. Chiral Monolithic Columns for Enantioselective Capillary Electrochromatography Prepared by in situ Copolymerization of a Monomer with Quinidine Functionality. 1. Optimization of Polymerization Conditions, Porous Properties, and Chemistry of Stationary Phase. Anal. Chem. 2000, 72 (19), 4614-4622. DOI: 10.1021/ac000322l
(12) Pell, R.; Lindner, W. Potential of Chiral Anion-Exchangers Operated in Various Subcritical Fluid Chromatography Modes for Resolution of Chiral Acids. J. Chromatogr. A 2012, 1245, 175–182. DOI: 10.1016/j.chroma.2012.05.023
(13) Horak, J.; Hofer, S.; Lindner, W. Optimization of a Ligand Immobilization and Azide Group Endcapping Concept via “Click Chemistry” for the Preparation of Adsorbents for Antibody Purification. J. Chromatogr. B 2010, 878 (32), 3382–3394. DOI: 10.1016/j.jchromb.2010.10.025
(14) Leitner, A.; Föttinger, A.; Lindner, W. Improving Fragmentation of Poorly Fragmenting Peptides and Phosphopeptides During Collision-Induced Dissociation by Malondialdehyde Modification of Arginine Residues. J. Mass Spec.2007, 42 (7), 950–959. DOI: 10.1002/jms.1233
(15) Lämmerhofer, M.; Nogueira, R.; Lindner, W. Multi-Modal Applicability of a Reversed-Phase/Weak Anion Exchange Material in Reversed Phase, Anion-Exchange, Ion Exclusion, Hydrophilic Interaction and Hydrophobic Chromatography Modes. Anal. Bioanal. Chem. 2011, 400, 2517–2530. DOI: 10.1007/s00216-011-4755-3
(16) Schuster, G.; Lindner, W. Chocolate HILIC Phase: Development and Characterization of Novel Saccharide-Based Stationary Phases by Applying Non-Enzymatic Browning (Maillard Reaction) on Amino Modified Silica Surfaces. Anal. Bioanal. Chem. 2011, 400, 2539–2554. DOI: 10.1007/s00216-011-4745-5
(17) Woiwode, U.; Reischl, R.; Buckenmaier, S.; Lindner, W.; Lämmerhofer, M. Imaging Peptide and Protein Chirality via Amino Acid Analysis by Enantioselective Chiral x Chiral Two-Dimensional Correlation Liquid Chromatography. Anal. Chem. 2018, 90 (13), 7963–7971. DOI: 10.1021/acs.analchem.8b00676
The Emerging Leader in Chromatography Award
Martina Catani is the 2024 recipient of the Emerging Leader in Chromatography Award, which recognizes the achievements and aspirations of a talented young separation scientist who has made strides early in their career toward the advancement of chromatographic techniques and applications.

Martina Catani is an assistant professor in Analytical Chemistry at the University of Ferrara in Italy. She received her PhD in chemistry in 2018 in Chemical Sciences from the University of Ferrara. Previously, she was a research associate in the Department of Chemistry and Pharmaceutical Sciences at the University of Ferrara and a postdoctoral research fellow at the Department of Chemistry and Applied Biosciences at the Institute for Chemical and Bioengineering at ETH Zurich in Switzerland. She was also a postdoctoral research fellow at the Department of Chemistry and Pharmaceutical Sciences at the University of Ferrara in Italy.
Catani received her bachelor’s degree in chemistry in 2011, magna cum laude, and master’s degree in chemical sciences in 2013, magna cum laude, from the University of Ferrara. Her PhD was completed in 2018 under the supervision of Alberto Cavazzini.
IMAGE 1: Martina Catani in the preparative LC lab at the University of Ferrara in September 2023 (All images courtesy of Martina Catani).

The University of Ferrara is one of the oldest universities in the world, founded in 1391. “Among the famous alumni who studied in Ferrara, it is worth mentioning Nicolaus Copernicus and Paracelsus,” she said.
Catani’s work primarily focuses on the fundamental aspects of kinetic and thermodynamic phenomena in both chiral and achiral high-performance liquid chromatography (HPLC) and supercritical fluid chromatography (SFC). Notably, she has made significant contributions to the field of separation science, earning the Csaba Horvath Award in 2018 at the HPLC meeting for the best research presented by a young scientist. Her research delves into the impact of kinetics, thermodynamics, and particle geometry on the efficacy of chiral and achiral columns in HPLC and SFC. Her studies have reinvigorated discussions on optimal particle characteristics for high-efficiency separations. Furthermore, she has actively developed advanced purification techniques for therapeutic bio-macromolecules using both single-column and continuous multicolumn preparative liquid chromatography.
Catani’s research group has a long tradition in the study of theoretical aspects of chromatographic techniques from many perspectives. “These perspectives range from the stochastic description of the chromatographic process to the study of competitive nonlinear systems by means of deterministic models,” she said.
In the past year, Catani’s group has started working on challenging research projects to reduce the environmental impact of chromatographic technologies. “We are working to replace the toxic solvents commonly used in LC (firstly, acetonitrile) with others that are more eco-friendly,” she said.
IMAGE 2: Martina Catani in the UHPLC-HRMS laboratory at the University of Ferrara in September 2023.

Career Highlights
Catani’s career in chromatography is marked by her significant contributions and practical applications in the field. She stands out for her collaborative research approach, working closely with industrial and academic partners to address real-world analytical challenges. Scientific discussions and idea-sharing are paramount. “The possibility to meet in person other colleagues and exhibitors from industries is, I believe, a fundamental and irreplaceable part of our work,” she said.
Catani has collaborated with major pharmaceutical companies like Fresenius Kabi iPSUM and Merck. Her work has involved the development of continuous purification methods for therapeutic peptides and the purification of carrier proteins for vaccines. These collaborations have had a meaningful impact on industry practices. In addition to her pharmaceutical collaborations, Catani has ventured into cannabinoid research in partnership with HPLC technology companies, such as ChromSword, Restek s.r.l., and Chiral Technologies. This expansion into new exploratory fields demonstrates her adaptability and willingness to explore emerging topics.
Catani has also filed a European patent application in collaboration with Fresenius Kabi iPSUM, demonstrating her innovation for advancing chromatography technology. Catani has also played a role in organizing scientific conferences, such as her participation in the organizing committee for HPLC 2019 in Milan, contributing to the success of the event.
With an impressive publication record of 53 articles and 3 book chapters, according to Scopus, Catani has consistently shared her knowledge and research findings with the chromatography community. As an active presenter at scientific conferences, she has delivered 35 presentations, including both invited and contributed talks, and has been a member of the Early-Career Board of Analytical Chemistry since 2022, reflecting her standing in the field. Her dedication to excellence was acknowledged with the Best PhD Thesis Award in Chemical Sciences at the University of Ferrara in 2018.
Catani is teaching, or has taught courses, named Instrumental Analytical Chemistry, Separation Methods, Analytical Chemistry Laboratory I and II, and Analytical Chemistry.
IMAGE 3: Martina Catani during her lecture at Balaton Symposium in 2023 (Siofok, Hungary, 4-6th September 2023).

Most Influential and Cited Research Work
Catani’s significant contributions in separation science are exemplified through five notable research works. In reference (18), Catani and associates examine the kinetic performance of columns packed with sub-2 µm C18 fully porous particles of narrow particle size distribution (nPSD). Their findings revealed that columns containing these particles exhibit low eddy dispersion and comparable longitudinal diffusion and mass transfer kinetics to other fully porous particles. Reference (19) showcases a study on chiral columns with Pirkle-type chiral selectors packed with either fully porous or core-shell particles. Notably, the authors achieved ultrafast separation of enantiomers within a second on a short column, emphasizing the correlation between kinetic performance and chiral selector loading.
IMAGE 4: Martina Catani and her colleagues at Balaton Symposium 2023 (Siofok, Hungary, 4-6th September 2023). From the left: Desiree Bozza (PhD student), Dr. Simona Felletti, Prof. Alberto Cavazzini, Greta Compagnin (PhD student), Dr. Tatiana Chenet, Dr. Chiara De Luca, Dr. Martina Catani.

Reference (20) reports a breakthrough in chiral separation efficiency using enantioselective supercritical fluid chromatography (SFC). By modifying a commercial SFC instrument, the authors harnessed the turbulent regime for unmatched kinetic performance, building on fundamental studies by chromatography pioneers. In reference (21), Felletti and associates explain the phenomenon of convex-upward van Deemter curves in chiral chromatography. The study elucidates this behavior’s origins in cases of strong analyte adsorption and negligible solid-phase diffusion. Reference (22) highlights a groundbreaking purification method for the peptidomimetic Icatibant using multicolumn countercurrent solvent gradient purification (MCSGP). This approach yielded remarkable gains in yield and productivity while reducing solvent consumption. These works collectively underscore Catani’s pivotal role in advancing separation science through innovative studies on kinetics, thermodynamics, and purification techniques, enhancing both understanding and practical applications in the field.
Catani’s studies include both chiral and achiral high performance liquid chromatography (HPLC) and supercritical fluid chromatography (SFC), which began in her PhD research studies. Her research aim was to unravel mass transfer phenomena through packed beds made of particles with different geometries and physico-chemical characteristics as well as to understand the thermodynamics of adsorption (adsorption isotherm determination) through nonlinear chromatography. “This work has been conducted in strict cooperation with Prof. Francesco Gasparrini’s group from University of Rome ‘La Sapienza,’” she said. Catani’s studies have culminated with the realization of ultrafast separations, “where the enantiomers of some compounds of pharmaceutical interest could be separated in less than one second with extremely high efficiency.”
Most Recent Research
The most recent work published by Catani and coworkers focus on a diverse range of topics in separation science. They include studies on the purification of Cannabigerol (CBG) from cannabis extracts using simulated moving bed chromatography, investigations into the retention behavior of small molecules and amino acids on chiral stationary phases, an exploration of rare convex-upward van Deemter curves in chiral liquid chromatography, the analysis of pesticide residues in water resources using multidimensional gas chromatography, and a review of the challenges and prospects for purifying cannabinoids from cannabis extracts. Additionally, Catani’s research extends to the synthesis of molecular hybrids with potential therapeutic applications, an examination of the effects of storage conditions on the phytochemical composition of garlic, and an overview of hydrophilic interaction liquid chromatography (HILIC) as an analytical technique. These recent articles collectively contribute to the advancement of separation science and its applications.
In Catani’s most recent publication, her research focused on purifying cannabigerol (CBG), a minor cannabinoid found in Cannabis sativa L., known for its various therapeutic properties (23). In this work, simulated moving bed chromatography was used to purify CBG from a cannabis extract efficiently and in an environmentally friendly manner. The method resulted in a CBG extract that was free of psychoactive tetrahydrocannabinol (THC) with a high recovery rate of 100% and a final purity of 97%.
In another recent article, Catani and coworkers explored the retention behavior of small molecules and N-protected amino acids on a zwitterionic teicoplanin chiral stationary phase (CSP) with different organic modifiers (24). This research explains that the choice of modifier significantly impacted efficiency and enantioselectivity. Methanol improved enantioselectivity, while acetonitrile allowed for high efficiency even at high flow rates.
Catani and colleagues investigated the rare occurrence of convex-upward van Deemter curves in chiral liquid chromatography (25). This work focuses on the behavior of chiral sulfoxides on a polysaccharide-based chiral stationary phase and explored the strong, localized adsorption of one enantiomer. The study explores correlations between molecular properties and specific interactions on the chiral stationary phase (CSP).
A chiral stationary phase (CSP) is a crucial element in chiral chromatography, involving a technique used to separate mirror-image isomers known as enantiomers. Enantiomers possess identical physical properties, but exhibit distinct chemical and biological behaviors. The CSP is typically composed of a chiral compound covalently bonded to a solid support or adhered to the surface of small particles within a chromatography column. Acting as the stationary phase, it remains fixed as samples containing enantiomers pass through. Enantiomers interact differently with the CSP due to their unique three-dimensional structures, resulting in varying retention times or elution sequences. This differential interaction enables the separation of enantiomers during chromatographic analysis.
In another study, Catani addressed analyzing the presence of pesticide residues in water resources due to agricultural and non-agricultural pesticide use (26). Her research team developed a method for analyzing 53 pesticides and their metabolites in surface and groundwater using multidimensional gas chromatography. The method was validated and applied to real-world water samples.
Multidimensional gas chromatography (MDGC) is an advanced analytical technique employed for the separation and analysis of complex mixtures of volatile compounds. It involves the sequential use of multiple chromatographic columns with different selectivity and separation mechanisms. In MDGC, the sample is first injected into the first-dimension column, where compounds are separated based on their volatility and polarity. The effluent from the first column is then transferred to a second-dimension column, often via a modulator, where further separation occurs, resulting in improved peak resolution. This multidimensional approach allows for the separation of closely eluting compounds and provides enhanced analytical specificity and sensitivity. MDGC is particularly valuable in applications such as environmental analysis, food and flavor profiling, and the characterization of complex mixtures in various scientific and industrial fields.
With the legalization of cannabis in many countries and increased research on its therapeutic properties, there is a growing need for highly purified cannabinoids. A review by Catani and team discusses the challenges and opportunities for purifying major and minor cannabinoids from cannabis extracts, especially for use as reference materials and in clinical trials (27).
The purification of major and minor cannabinoids from cannabis extracts holds significant importance for several reasons. Firstly, it allows for production of standardized and highly pure cannabinoid compounds, which are crucial for scientific research, clinical trials, and the development of pharmaceutical products. These purified compounds serve as reference materials, enabling accurate and reproducible experiments to assess the therapeutic properties and potential medical benefits of cannabinoids. Additionally, the precise purification of cannabinoids ensures compliance with regulatory requirements for pharmaceutical and therapeutic applications, where quality control and consistency are paramount. Moreover, the purification process removes unwanted impurities and psychoactive components like THC, making the resulting cannabinoid extracts safer for both research and medical use. The purification of major and minor cannabinoids not only facilitates scientific advancements, but also enhances the safety and reliability of cannabinoid-based therapies and products.
Another recent research article by Catani and associates explores the synthesis of molecular hybrids by connecting bioactive molecules with a metabolizable linker. Specifically, it describes the lipase-catalyzed condensation of ascorbic acid with ketone bodies, which results in novel compounds with potential for treating neurodegenerative diseases and cardiac injuries (28). This study focused on creating molecular hybrids by connecting bioactive molecules using a metabolizable linker for the treatment of complex diseases. It successfully synthesized two novel compounds, 6-O-acetoacetyl ascorbic acid and 6-O-(R)-3-hydroxybutyryl ascorbic acid, using a lipase-catalyzed method. The research not only provided efficient synthesis pathways but also characterized the compounds and explored their antioxidant properties, offering valuable insights for potential therapeutic applications.
IMAGE 5: Martina Catani and her colleagues at her wedding party (Ferrara, 27th May 2023). From the left: (Standing) Desiree Bozza (PhD student), Dr. Tatiana Chenet, Dr. Elena Sarti, Dr. Denise Bellotti, Dr. Natasha Spadafora, Matteo Spedicato (PhD student), Dr. Giulio Lievore, Dr. Claudia Stevanin, Dr. Martina Catani; (Crouching) Alessandro Buratti (PhD student), Prof. Alberto Cavazzini.

Catani and coworkers investigated the effect of storage conditions on the phytochemical composition, biological effects, and shelf-life of Voghiera garlic, a Protected Designation of Origin (PDO) variety (29). Different storage conditions were considered, and changes in organosulfur compounds, total condensed tannins, flavonoids, phenolic compounds, and antioxidant activity were monitored. The study also assessed the impact of storage on the garlic’s bioactive effects. The research conducted by Catani and colleagues holds significant importance as it addresses the crucial issue of preserving the quality and bioactive properties of Voghiera garlic. By systematically investigating various storage conditions, the study offers insights into the optimal methods to retain the garlic’s phytochemical composition, including vital organosulfur compounds, tannins, flavonoids, and phenolic compounds. Moreover, by assessing changes in antioxidant activity and bioactive effects during storage, the research provides valuable guidance for maintaining the garlic’s bioactive potency, shelf life, and overall quality, which is essential for its culinary and potential therapeutic applications. This information contributes to the preservation and utilization of a valuable agricultural product, benefiting both consumers and the garlic industry.
A book chapter by Catani provides an overview of hydrophilic-interaction chromatography (HILIC), highlighting its characteristics and applications (30). The chapter discusses the fundamentals of retention mechanisms in HILIC, and the materials used for stationary and mobile phases, emphasizing HILIC’s role as a complementary chromatographic technique for separating polar molecules. Hydrophilic-interaction chromatography (HILIC) is a chromatographic technique of great importance in analytical chemistry for several reasons. Firstly, it enables the separation of highly polar and hydrophilic compounds, such as small polar metabolites, peptides, and glycoproteins, which are often challenging to separate using traditional reversed-phase chromatography. This capability is crucial in fields like metabolomics and proteomics, where the analysis of these compounds is essential for understanding biological processes and disease mechanisms. Secondly, HILIC is valuable in environmental and pharmaceutical analysis, where the separation of polar analytes is common. Additionally, HILIC can be used in tandem with other chromatographic methods, enhancing its versatility in comprehensive analyses. HILIC plays a vital role in expanding the range of analytes that can be effectively separated and detected in various scientific and industrial applications, contributing significantly to advances in analytical chemistry.
A Bright Future
Martina Catani has demonstrated remarkable potential for a bright future in the field of chromatography and separation science. Her ability to quickly grasp complex concepts, as evidenced during her research visits to leading institutions, showcases her adaptability and determination.
“Sustainable development is the most important challenge that we have to face in all fields,” she said. “I may anticipate that there will be an increasing interest in ‘green’ chromatography. This will embrace both the study of the fundamentals of separation and the development of new applications to satisfy different requests.”
Catani’s career is characterized by her pragmatic approach to chromatography, her impactful collaborations, and her dedication to advancing the field. Her contributions, both in research and practical applications, continue to make a meaningful impact on chromatography. Catani’s strong network and active involvement in collaborative projects and academic events underline her dedication to advancing the field. She has already achieved an impressive research output, with an h-index of 13 and numerous presentations at international conferences. Her enthusiasm, presentation skills, and sociable nature contribute to her standing as a promising scientist and future leader in chromatography. Her impactful research in macromolecule and optical isomer separation, garnering attention from the pharmaceutical industry, further solidifies her potential for excellence in the field. Catani’s comprehensive knowledge and dedication make it highly likely for her to continue to significantly contribute and lead the advancement of separation science.
References
(1) Catani, M.; Ismail, O. H.; Cavazzini, A.; Ciogli, A.; Villani, C.; Pasti, L.; Bergantin, C.; Cabooter, D.; Desmet, G.; Gasparrini, F.; Bell, D. S. Rationale Behind the Optimum Efficiency of Columns Packed with New 1.9 µm Fully Porous Particles of Narrow Particle Size Distribution. J. Chromatogr. A 2016,1454, 78–85. DOI: 10.1016/j.chroma.2016.05.037
(2) Ismail, O. H.; Pasti, L.; Ciogli, A.; Villani, C.; Kocergin, J.; Anderson, S.; Gasparrini, F.; Cavazzini, A.; Catani, M. Pirkle-Type Chiral Stationary Phase on Core–Shell and Fully Porous Particles: Are Superficially Porous Particles Always the Better Choice toward Ultrafast High-Performance Enantioseparations? J. Chromatogr. A 2016, 1466, 96–104. DOI: 10.1016/j.chroma.2016.09.001
(3) Ismail, O. H.; Losacco, L.; Mazzoccanti, G.; Ciogli, A.; Villani, C.; Catani, M.; Pasti, L.; Anderson, S.; Cavazzini, A.; Gasparrini, F. Unmatched Kinetic Performance in Enantioselective Supercritical Fluid Chromatography by Combining Latest Generation Whelk-O1 Chiral Stationary Phases with a Low-Dispersion In-House Modified Equipment. Anal. Chem. 2018, 90, 10828–10836. DOI: 10.1021/acs.analchem.8b01907
(4) Felletti, S.; De Luca, C.; Lievore, G.; Chenet, T.; Chankvetadze, B.; Farkas, T.; Cavazzini, A.; Catani, M. Shedding Light on Mechanisms Leading to Convex-Upward Van Deemter Curves on a Cellulose Tris(4-chloro-3-methylphenylcarbamate)-Based Chiral Stationary Phase. J. Chromatogr. A 2020, 1630, 461532. DOI: 10.1016/j.chroma.2020.461532
(5) De Luca, C.; Felletti, S.; Bozza, D.; Lievore, G.; Morbidelli, M.; Sponchioni, M.; Cavazzini, A.; Catani, M.; Cabri, W.; Macis, M.; Ricci, A. Process Intensification for the Purification of Peptidomimetics: The Case of Icatibant through Multicolumn Countercurrent Solvent Gradient Purification (MCSGP). Ind. Eng. Chem. Res. 2021, 60, 6826–6834. DOI: 10.1021/acs.iecr.1c00520
(6) De Luca, C.; Krauke, Y.; Stephan, S.; Greco, G.; Compagnin, G.; Buratti, A.; Cavazzini, A.; Catani, M.; Felletti, S. Green Cannabigerol Purification Through Simulated Moving Bed Chromatography. Green Anal. Chem. 2023, 6 (1), 100066. DOI: 10.1016/j.greeac.2023.100066
(7) Felletti, S.; De Luca, C.; Mazzoccanti, G.; Gasparrini, F.; Manetto, S.; Franchina, F.A.; Chenet, T.; Pasti, L.; Cavazzini, A.; Catani, M. Understanding the Transition from High-Selective to High-Efficient Chiral Separations by Changing the Organic Modifier with Zwitterionic-Teicoplanin Chiral Stationary Phase. Anal. Chem. 2023, 95 (25), 9630–9637. DOI: 10.1021/acs.analchem.3c01344
(8) Catani, M.; Cavazzini, A.; De Luca, C.; Felletti, S. When van Deemter Goes Upside Down in Chiral Chromatography. LCGC Supplements, Advances in (U)HPLC 2023, 36, (s5), 20–23. DOI: 10.56530/lcgc.eu.st5666h2
(9) Romagnoli, M.; Scarparo, A.; Catani, M.; Giannì, B.; Pasti, L.; Cavazzini, A.; Franchina, F. A. Development and Validation of a GC×GC-ToFMS Method for the Quantification of Pesticides in Environmental Waters. Anal. Bioanal. Chem. 2023, 415, 4545–4555. DOI: 10.1007/s00216-023-04686-8
(10) Felletti, S.; Compagnin, G.; Krauke, Y.; Stephan, S.; Greco, G.; Buratti, A.; Chenet, T.; De Luca, C.; Catani, M.; Cavazzini, A. LCGC Europe, 2023, 36, (04), 122–131. DOI: 10.56530/lcgc.eu.jp5571c5
(11) Venturi, V.; Lerin, L.A.; Presini, F.; Giovannini, P.P.; Catani, M.; Buratti, A.; Marchetti, N.; Dilliraj, L.N.; Aprile, S. Enzymatic Synthesis of Ascorbic Acid-Ketone Body Hybrids. Catalysts2023, 13 (4), 691. DOI: 10.3390/catal13040691
(12) Tedeschi, P.; Brugnoli, F.; Merighi, S.; Grassilli, S.; Nigro, M.; Catani, M.; Gessi, S.; Bertagnolo, V.; Travagli, A.; Caboni, M.F.; Cavazzini, A. The Effect of Different Storage Conditions on Phytochemical Composition, Shelf-Life, and Bioactive Compounds of Voghiera Garlic PDO. Antioxidants 2023, 12 (2), 499. DOI: 10.3390/antiox12020499
(13) Cavazzini, A.; Catani, M.; Felinger, A. Hydrophilic Interaction Liquid Chromatography. In Liquid Chromatography, Elsevier 2023, pp. 227–249. DOI: 10.1016/B978-0-323-99968-7.00030-8
About the Author
Jerome Workman, Jr. serves on the Editorial Advisory Board of Spectroscopy and is the Senior Technical Editor for LCGC and Spectroscopy. He is the co-host of the Analytically Speaking podcast and has published multiple reference text volumes.


Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Characterizing Polyamides Using Reversed-Phase Liquid Chromatography
May 5th 2025Polyamides can be difficult to characterize, despite their use in various aspects of everyday life. Vrije Universiteit Amsterdam researchers hoped to address this using a reversed-phase liquid chromatography (RPLC)-based approach.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)