Increasing Speed for Biocharacterizations: Peptide Mapping ofMonoclonal Antibodies with an Agilent AdvanceBio Peptide Mapping Column
The Application Notebook
Agilent Technologies
Peptide mapping by reversed-phase chromatography is the mainstay technique in biotherapeutic analysis, delivering the comprehensive characterization of biopharmaceutical products. When interfaced with a mass spectrometer (MS), it can deliver the identification of proteins and its variants, determine post-translational modifications (PTMs) and locations, and confirm protein sequences.
However, peptide mapping represents a significant chromatographic challenge because of the inherent complexity of protein digests. As a result, many organizations struggle with developing robust and reliable peptide maps. In general, their peptide maps have suffered from low sensitivity, poor peak shapes, and very long separation times to achieve the desired resolution.
The new 2.7 um AdvanceBio Peptide Mapping column has been introduced to fulfill a critical segment in biotherapeutic characterizations, for generating both rapid and highly efficient peptide maps at low liquid chromatography (LC) system pressures. Using superficially porous chromatographic media, the AdvanceBio Peptide Mapping columns achieve substantial improvements in peptide mapping during very fast run times, while still maintaining high efficiency peak performance.

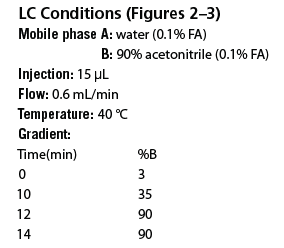
Figure 1: Fast HPLC optimizations of mAb tryptic digest. Mobile phase A: water (0.1% FA) Mobile phase B: 90% acetonitrile (0.1% FA), Injection: 15 µL, Temperature: 40 °C, DAD: 215 nm (a): 75 min. separation on a 2.1 à 150 mm AdvanceBio Peptide Mapping column generating 59 peptide peaks. (b): 14 min. separation on a 2.1 à 100 mm AdvanceBio Peptide Mapping column generating 57 peptide peaks.
Rapid mAb Tryptic Digest Peptide Mapping by HPLC–UV
To highlight this performance, the AdvanceBio Peptide Mapping column was used for the rapid LC–MS analysis of a monoclonal antibody (mAb) tryptic digest. Traditionally, shallow gradients of 2 h or longer are employed for mapping mAb peptide maps to achieve the desired baseline performance. The longer run times ensure that optimum resolution is achieved and that critical PTM peak information is not sacrificed during the shorter run. However, the AdvanceBio Peptide Mapping column has the flexibility to increase analysis speed without sacrificing separation performance. The UV–LC AdvanceBio column separations in Figure 1 demonstrate this flexibility. The top chromatogram provides an example of a more common peptide map generated during 75 min on a 2.1 × 150 mm column at a standard flow rate (relative to column internal diameter) of 0.2 mL/min. The separation provides excellent baseline resolution with 59 mAb tryptic digest peaks resolved over the entire gradient profile. In the bottom chromatogram comparison, a 2.1 × 100 mm AdvanceBio column was used with a very steep gradient at a high flow rate of 0.6 mL/min but at a fraction of the runtime in under 14 min. In this separation, the mAb tryptic peak count has remained relatively unchanged (57 peaks) while baseline resolution, peak shapes, sensitivity, and selectivity have not been compromised. In addition, the column back pressure remained well under 500 bar making this rapid and highly efficient separation achievable on traditional high perfomance liquid chromatography (HPLC) instrumentation.

Delivering UHPLC-like Performance Without the High Back Pressure Constraints
Peptide mapping by ultrahigh-pressure liquid chromatography (UHPLC) can provide the basis for a more detailed characterization for protein biotherapeutics taking advantage of smaller particles packed in shorter column lengths to achieve high efficiency maps during faster run times. However, the pressure requirement for these separations becomes increasingly high, thereby preventing operation on traditional 400 and 600 bar HPLC instruments and severely limiting its broader application. The new 2.7 um AdvanceBio Peptide Mapping column can generate UHPLC-like mapping performance while providing the flexibility to use with UHPLC or HPLC instrumentation.

Figure 2: (a) AdvanceBio Peptide Mapping column and (b) UHPLC peptide mapping column total ion chromatograms (TIC) for a mAb tryptic digest.
Figure 2 displays the reversed-phase LC–MS separation performance between an AdvanceBio Peptide Mapping column and a competitor UHPLC peptide mapping column. The total ion chromatogram (TIC) comparisons clearly detail very comparable resolving performance and indicate the ability of the AdvanceBio peptide columns to adequately and efficiently resolve a mAb tryptic digest to deliver comparable performance to the UHPLC–MS separation at HPLC traditional pressure (433 bar).

Figure 3: Extracted compound chromatograms (ECC) for a mAb tryptic digest. (a) AdvanceBio Peptide Mapping column, 433 bar yielding 99.63% sequence coverage. (b) UHPLC peptide mapping column, 700 bar yielding 99.0% sequence coverage.
In the extracted compound chromatogram (ECC) (Figure 3) the AdvanceBio Peptide Mapping HPLC–MS analysis delivered excellent sequence coverage performance in comparison to the UHPLC–MS column analysis. Sequence coverage for the AdvanceBio column was 99.63%, while the UHPLC column delivered 99.0%. The flexibility to obtain UHPLC-like results on the AdvanceBio peptide mapping column, without the pressure constraints of UHPLC, can make this column a more attractive and universal choice for rapid and efficient characterization of biotherapeutics.
Conclusions
The AdvanceBio Peptide Mapping column reduced the analysis time for reversed-phase LC–MS peptide mapping of a monoclonal antibody (mAb) tryptic digest. In combination with optimized short gradient conditions, this application describes methods for generating rapid (14 min) and highly efficient mAb peptide maps requiring only low LC pressure operation (<450 bar). Alternatively, a UHPLC peptide mapping column was compared for rapid mapping performance with the AdvanceBio Peptide Mapping column. In this evaluation, the AdvanceBio Peptide Mapping column delivered well-resolved peptide peaks across the entire gradient profile comparatively and resulted in high sequence coverage of the mAb. However, the AdvanceBio Peptide Mapping separations were generated at low system pressures, highlighting important flexibility for operating on either HPLC or UHPLC instrumentation.

Agilent Technologies
2850 Centerville Road, Wilmington, Delaware 19808, USA
Website: www.agilent.com/chem/advancebio/

Separating Impurities from Oligonucleotides Using Supercritical Fluid Chromatography
February 21st 2025Supercritical fluid chromatography (SFC) has been optimized for the analysis of 5-, 10-, 15-, and 18-mer oligonucleotides (ONs) and evaluated for its effectiveness in separating impurities from ONs.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









