In situ Hydrogenolysis with 3D-Printed Jet and Flame Ionization Detection for Carbon Oxides and Volatile Aldehydes Analysis
An effective analytical strategy based on post-column in situ hydrogenolysis and flame ionization has been successfully developed to characterize carbon oxides and volatile aldehydes in various matrices. The analytes were chemically converted to their corresponding hydrocarbon analogs with an appropriate catalyst embedded within the jet assembly of the detector. The augmentation of this approach substantially improves method performance and preserves both system flexibility and robustness, with facile conversion between conventional jets and catalytic-based jets for added capability when required.
Carbon monoxide (CO) and carbon dioxide (CO2) are chemicals of high significance within numerous industries (1–5). Carbon monoxide gained recognition as an invaluable reagent in the 1900s (6,7). Three industrial processes illustrate its evolution in the industry: the Fischer-Tropsch process, where coal and related carbon-rich feedstocks are converted into liquid fuels via CO; the hydroformylation process where a mixture of CO and hydrogen was combined with olefins to produce aldehydes; and the Cativa process, where CO combines with methanol to create acetic acid. The quest for sustainable energy involves the production of fuels such as methanol from CO2. From an environmental perspective, the measurement of CO2 is a critical analysis, as CO2 is a known greenhouse gas.
Volatile aliphatic aldehydes are also chemicals of significance. Formaldehyde is used to produce industrial resins for multiple applications (8–11). Acetaldehyde is a feedstock to produce acetic acid and can be used as a precursor to the formation of alkyd resins (12–14). Propionaldehyde is predominantly used as a precursor to trimethylolethane. Acrolein is an intermediate in the Skraup synthesis of quinolines. Volatile aldehydes are present as ubiquitous air pollutants.
Furthermore, these compounds can be residual reactants or impurities from various chemical processes. These aldehydes can also negatively impact human health due to their volatility, toxicity, and widespread use. Formaldehyde is a known irritant, and it possesses the potential to react with hydrochloric acid to form bis-chloromethyl ether, which is a known human carcinogen (15–18). Acetaldehyde is classified as a Group I carcinogen by the International Agency for Research on Cancer (18,19). Acrolein is toxic and is a strong irritant for the skin and eyes. Amongst the four aldehydes, propionaldehyde exhibits lower acute toxicity.
Many techniques for the measurement of carbon oxides are available. A popular strategy with single-digit parts-per-million level carbon oxides detection is post-column reaction gas chromatography and flame ionization detection (FID). Catalytic reduction with nickel of carbon oxides to methane for detection by FID was first described by Porter and Volman (20). Analytical devices based on this approach, known as the “methanizer”, have been made commercially available for many decades as a stand-alone or integrated chromatographic tool (21–23). While the device functions relatively well, reliability can be an issue, and extra band broadening is often observed with the added void-volume. Also, additional hardware is required for implementation. An obvious constraint is the incorporation of the supporting utilities that can reduce the flexibility of the analytical platform.
Meanwhile, a variety of techniques for the measurement of highly volatile aliphatic aldehydes have been reported in the literature, including high-performance liquid chromatography (HPLC), gas chromatography (GC), and chemical sensors, to name a few (24–31). Each technique has its strengths and constraints. GC and HPLC face challenges in performing direct detection, particularly for formaldehyde.
This article introduces an analytical strategy for directly measuring carbon oxides and volatile aldehydes in gas phase matrices without sample pretreatment or pre-concentration. In addition, we demonstrated that carbon oxides and volatile aldehydes can be converted to their corresponding hydrocarbon analogs reliably and detected by FID at a concentration level as low as 1 pg C/s (32–34).
Experimental
An Agilent 7890A GC (Agilent Technologies), equipped with a split/splitless inlet operating in split mode and an FID, was employed for the carbon oxide analysis, while an Agilent 8890A GC (Agilent), with a similar configuration, was used for the volatile aldehydes analysis.
A prototype, 3D-printed steel jet assembly with embedded catalyst was provided by Activated Research Company. Activated Research Company has commercialized the 3D-printed jet technology as the Jetanizer. The Jetanizer has dimensions equivalent to those of a classical FID jet employed by the 7890A and 8890A gas chromatographs with a length of 43 mm and a jet orifice of 0.29 mm. One mL manual injections were conducted using a one mL Hamilton Gastight syringe (Hamilton Company) for the gas samples.
For the separation of CO and CO2 in various matrices, a 30 m × 0.53 mm id GS-Q (Agilent) porous layer open tubular column was used. A 15 m × 0.53 mm id MS-13X column (Restek) was used for CO analysis where percent level of oxygen is present in the matrix with oven temperature was set isothermally at 100 °C. The carrier gas was hydrogen at a column flow rate of 5 mL/min. The temperature was programmed from 40 °C (2 min) to 150 °C at 15 °C/min (2 min).
A 30 m × 0.32 mm-i.d. × 0.26μm SLB-IL60i (Sigma-Aldrich) column was used to separate the aldehydes from other components in the various matrices. The carrier gas was helium at a 2.5 mL/min column flow rate. A split/splitless inlet was equipped with a 4 mm ultra-inert liner, operating at 200 °C in split mode at a split ratio 5:1. The temperature was programmed from 70 °C (0.5 min) to 150 °C at 15 °C/min and maintained at 150 °C for 2 min.
In both cases, the column was connected to a 0.5 m × 0.53 mm i.d. Pro- Steel (Agilent) uncoated deactivated metal tubing with Ultimate Union (Agilent) and SilTite (Trajan Medical) ferrules. The FID temperature was 400 °C, with a hydrogen flow at 30 mL/min, a nitrogen flow at 20 mL/min, and an air flow at 350 mL/min.
Data were collected with Agilent ChemStation software version B.04.03SP1 for the carbon oxides analysis and version C.01.10 for the aldehyde analysis.
A test mixture that contains 0.1% of carbon monoxide, methane, carbon dioxide, ethylene, acetylene, ethylene, propylene, and propane in argon and a test mixture that contains 0.1% of straight-chain hydrocarbons including methane, ethane, propane, butane, pentane, and hexane obtained from Air Liquide were used for method development.
Formaldehyde as formalin solution (37 wt% in water, containing 10–15% methanol as stabilizer), acetaldehyde, propionaldehyde, and acrolein were purchased from Sigma-Aldrich. The stock standards were prepared by injecting two μL of formaldehyde and one μL of other aldehydes into a 1000 mL nitrogen-purged glass vessel (Sigma-Aldrich). The standards were heated to 50 °C to facilitate complete analyte evaporation, and the vessel was allowed to reach equilibrium at ambient temperature for 1 h before analysis. With this method, a standard of 596 ppm (v/v) formaldehyde, 433 ppm (v/v) acetaldehyde, 337 ppm (v/v) propionaldehyde, and 362 ppm (v/v) acrolein in nitrogen can be prepared. Serial dilution with nitrogen generated standards for calibration and detection limit studies.
Results and Discussions
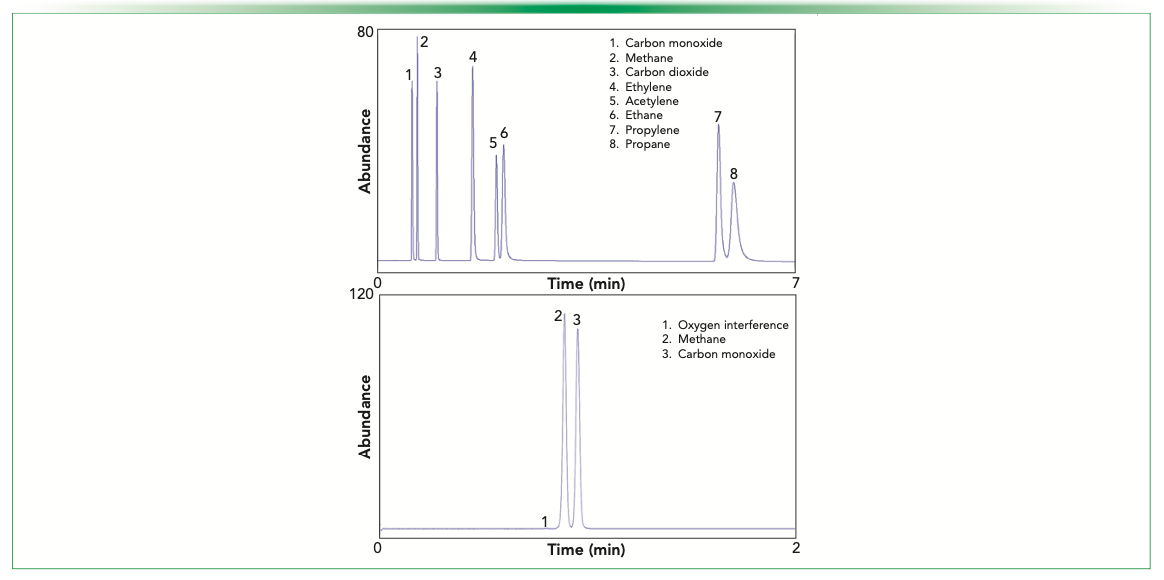
The capability to conduct in situ hydrogenolysis inside a conventional FID jet with a catalyst affords the implementation of post-column reaction gas chromatography without the need for additional hardware. As we reported earlier, the modified jet’s slight increase in back pressure can be employed to conduct post-column backflush using FID fuel gas to reduce analytical time, keep the jet from being contaminated, and protect the analytical system integrity (32,33). Optimization of performance has been reported elsewhere (32–34). CO and CO2 can be separated from different matrices using divinyl benzene as a stationary phase. However, oxygen is co-eluted with CO in this type of stationary phase. If the sample matrix contains a certain percent level of oxygen, the oxygen can react with residual carbon deposited on the catalyst surface to generate CO, resulting in a false positive result. For trace analysis of CO in the presence of percent level of oxygen, a viable solution involves using a molecular sieve 13X column. Here, oxygen is well-separated from CO, completely powered by eliminating the interference caused by oxygen. Figure 1a shows the separation of CO and CO2 from other hydrocarbons with the divinyl benzene column, while Figure 1b shows the separation of 0.1% methane and CO with the MS-13X column.
FIGURE 1: (a) Separation of carbon monoxide and carbon dioxide from hydrocarbons with GS-Q column. (b) Separation of carbon monoxide and methane from oxygen interference at 100 °C with MS-13X column. Compound names are labeled within subfigures.
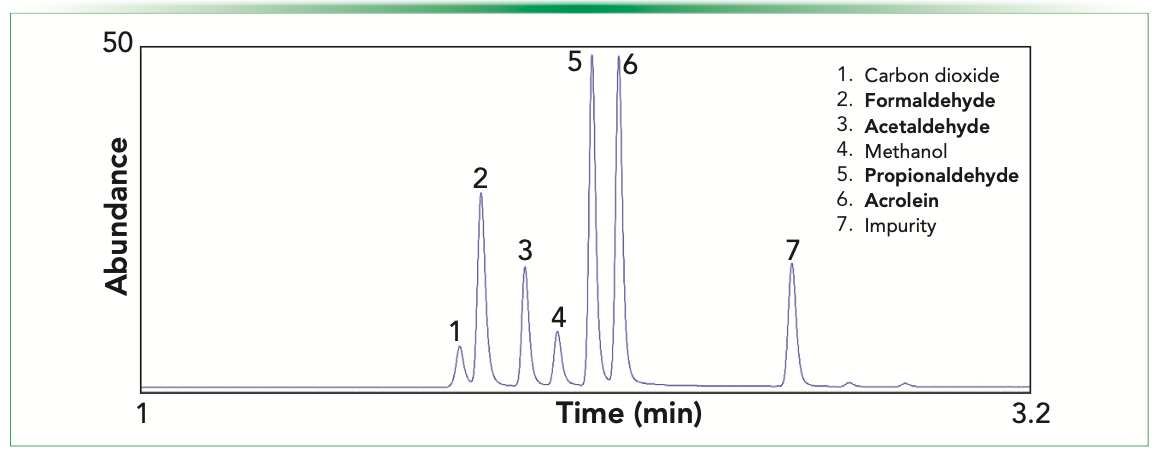
We reported the optimized conditions for aldehyde analysis earlier (34). A substantial gain in sensitivity for formaldehyde with the modified jet as formaldehyde was converted to methane for detection. For formaldehyde, an improvement in area count of more than 35 times was observed. This improvement demonstrated the advantage of detecting formaldehyde with the 3D-printed jet. As predicted, with the increased carbon chain length of aldehydes, which increased CHO+ ions formation with conventional FID, the effect of hydrogenolysis on the gain in FID response gradually diminished.
Figure 2 shows a chromatogram of formaldehyde, acetaldehyde, propionaldehyde, and acrolein standard at a concentration of 596 ppm (v/v), 433 ppm (v/v), 337 ppm (v/v), 362 ppm (v/v), respectively demonstrating the excellent chromatographic fidelity.
FIGURE 2: Separation of volatile aldehydes with SLB-IL60i stationary phase.
While the jet assembly was demonstrated to be robust, it has been reported that certain compounds can negatively impact the catalyst, such as active sulfur compounds, unsaturates or heavier aromatics.
Conclusions
A novel analytical technique that employs in situ hydrogenolysis with flame ionization detection for measuring carbon oxides and volatile aldehydes in various matrices has been successfully developed and implemented. Moreover, the strategy offers low cost-of-ownership with the jet being a disposable component and straightforward to implement.
Acknowledgments
Drs. Naoko Akiya, Jaime Curtis-Fisk, Wayde Konze, and Tonya Stockman of Dow are acknowledged for their support. Dr. Matthias Pursch, also of Dow, is recognized for his help in reviewing the manuscript. Dr. Andrew Jones of Advanced Research Inc. is acknowledged for providing the prototype modified jet assembly for technology development and fruitful discussions. Drs. Len Sidisky of Sigma-Aldrich and Daniel Armstrong of the University of Texas in Arlington were acknowledged for their innovations in using ionic liquid as a stationary phase and for their invaluable guidance.
References
(1) Orr, F. M.; Taber, J. J. Use of Carbon Dioxide in Enhanced Oil Recovery. Science 1984, 224, 563–569. DOI: 10.1126/science.224.4649.563
(2) Schroeder, G.; Biondi, J.; Johnston, D. Pneumatic System. US Patent US5664563A, 1997.
(3) Seshadri, G.; Lin, C.; Bocarsly, A. A New Homogeneous Electrocatalyst for the Reduction of Carbon Dioxide to Methanol at Low Overpotential. J. Electroanal. Chem. 1994, 372, 145–150. DOI: 10.1016/0022-0728(94)03300-5
(4) Daniels, J. A.; Krishnamurthi, R.; Rizvi, S. H. S. A Review of Effects of Carbon Dioxide on Microbial Growth and Food Quality. J. Food Prot. 1985, 48, 532–537. DOI: 10.4315/0362-028X-48.6.532
(5) Santamouris, M.; Synnefa, A.; Assimakopoulos, M.; Livada, I.; Pavlou, K.; Pagalastra, M.; Gaitani, N.; Kolokotsa, D.; Assimakopoulos, V. On the Impact of Urban Heat Island and Global Warming on the Power Demand and Electricity Consumption of Buildings—A Review. Energy Build. 2008, 40, 1833–1843. DOI: 10.1016/j.enbuild.2014.09.052
(6) Bierhals, J. Carbon Monoxide. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH, 2001. DOI: 10.1002/14356007.a05_203
(7) Tang, X.; Bai, Y.; Duong, A.; Smith, M.; Li, L.; Zhang, L. Formaldehyde in China: Production, Consumption, Exposure Levels, and Health Effects. Environ. Int. 2009, 35, 1210–1224. DOI: 10.1016/j.envint.2009.06.002
(8) Akyüz, K. C.; Nemli, G.; Baharoglu, M.; Zekoviç, E. Effects of Acidity of the Particles and Amount of Hardener on the Physical and Mechanical Properties of Particleboard Composite Bonded with Urea Formaldehyde. Int. J. Adhes. Adhes. 2010, 30, 166–169. DOI: 10.1016/j.ijadhadh.2009.12.006
(9) Chang, C.; Lin, K. Assessment of the Strategies for Reducing VOCs Emission from Polyurea-Formaldehyde Resin Synthetic Fiber Leather Industry in Taiwan. Resour. Conserv. Recycl. 2006, 46, 321–334. DOI: 10.1016/j.resconrec.2005.08.007
(10) Greenfield, S. A.; Dupont, J. A. Formaldehyde Stabilized Coating Compositions. US Patent US4129448A, 1973.
(11) Yoneda, N.; Kusano, S.; Yasui, M.; Pujado, P.; Wilcher, S. Recent Advances in Processes and Catalysts for the Production of Acetic Acid. Appl. Catal., A 2001, 221, 253–265. DOI: 10.1016/S0926-860X(01)00800-6
(12) Jones, J. H. The Cativa Process for the Manufacture of Acetic Acid. Platinum Met. Rev. 2000, 44, 94–105.
(13) Salthammer, T. The Formaldehyde Dilemma. Int. J. Hyg. Environ. Health 2015, 218, 433–436. DOI: 10.1016/j.ijheh.2015.02.005
(14) Duong, A.; Steinmaus, C.; McHale, C. M.; Vaughan, C. P.; Zhang, L. Reproductive and Developmental Toxicity of Formaldehyde: A System Review. Mutat. Res., Rev. Mutat. Res. 2011, 728, 118–138. DOI: 10.1016/j.mrrev.2011.07.003
(15) Nurmatov, U. B.; Tagiyeva, N.; Semple, S.; Devereux, G.; Sheikh, A. Volatile Organic Compounds and Risk of Asthma and Allergy: A Systematic Review. Eur. Respir. Rev. 2015, 24, 92–101. DOI: 10.1183/09059180.00000714
(16) Liebling, T.; Rosenman, K. D.; Pastides, H.; Griffith, R. G.; Lemeshow, S. Cancer Mortality among Workers Exposed to Formaldehyde. Am. J. Ind. Med. 1984, 5, 423–428. DOI: 10.1002/ajim.4700050602
(17) Salaspuro, M. Acetaldehyde and Gastric Cancer. J. Dig. Dis. 2011, 12, 51–59. DOI: 10.1111/j.1751-2980.2011.00480.x
(18) Soffritti, M.; Belpoggi, F.; Lambertin, L.; Lauriola, M.; Padovani, M.; Maltoni, C. Results of Long-Term Experimental Studies on the Carcinogenicity of Formaldehyde and Acetaldehyde in Rats. Ann. N. Y. Acad. Sci. 2002, 982, 87–105. DOI: 10.1111/j.1749-6632.2002.tb04926.x
(19) Lachenmeier, D. W.; Kanteres, F.; Rehm, J. Carcinogenicity of Acetaldehyde in Alcoholic Beverages: Risk Assessment Outside Ethanol Metabolism. Addiction 2009, 104, 533–550. DOI: 10.1111/j.13600443.2009.02516.x
(20) Porter, V.; Volman, D. H. Flame Ionization Detection of Carbon Monoxide for Gas Chromatographic Analysis. Anal. Chem. 1962, 34, 748–749. DOI: 10.1021/ac60187a009
(21) Luong, J.; Sieben, L.; Dahoy, T.; de Zeeuw, J. Proceedings of the 19th International Symposium on Capillary Chromatography and Electrophoresis, Wintergreen, VA, USA, May 18–22, 1997; Poster #38; Lee, M., Ed.
(22) Li, X.; Guan, Z. Analysis of Permanent Gases and Light Hydrocarbons Using Agilent 7820A GC with 3-Valve System. Agilent Publication 5990-4667EN; October 13, 2009. https://www.agilent.com/cs/library/applications/5990-4667EN.pdf
(23) Lamb, A.; Larson, K. A.; Tollefson, E. L. A Gas Chromatographic Method for Exhaust Gas Analysis. JAPCA 1973, 23, 200–202. DOI: 10.1080/00022470.1973.10469766
(24) Wang, C. Simultaneous Analysis of Greenhouse Gases by Gas Chromatography. Agilent Technologies Publication #5990-5129EN, January 15, 2010. https://www.chem-agilent.com/pdf/5990-5129EN.pdf
(25) de Zeeuw, J.; Oden, K.; Burger, B.; Sellers, K. A Simple Solution for Permanent Gas Analysis by Gas Chromatography Using a FID/Methanizer Detection. In PittCon Conference & Expo 2017, Pittsburgh, PA, USA, March 6–9, 2017.
(26) Duvekot, C. Analysis of Low ppm CO and CO2 with CO/CO2 Analyzer, Varian Application Note #SI-02091, May 2010. https://gcms.cz/labrulez-bucket-strapi-h3hsga3/paper/SI-02091.pdf
(27) Maduskar, S.; Teixeira, A. R.; Paulsen, A. D.; Krumm, C.; Mountziaris, T. J.; Fan, W.; Dauenhauer, P. J. Quantitative Car- bon Detector (QCD) for Calibration-Free, High-Resolution Characterization of Complex Mixtures. Lab Chip 2015, 15, 440–447. DOI: 10.1039/C4LC01180E
(28) Schomburg, G. Gas Chromatography; VCH, 1990; pp 150–153.
(29) Schneider, W.; Frohne, J.; Bruderreck, H. Selektive Gaschromatographische Messung Sauerstoffhaltiger Verbindungen Mittels Flammenionisationsdetektor. J. Chromatogr. A 1982, 245, 71–83. DOI: 10.1016/S0021-9673(00)82476-0
(30) Kaiser, R. E.; Rider, A. R. In Proceedings of the 4th International Clean Air Congress, Tokyo, May 1977; pp 451–460.
(31) Huffman, E. W. D., Jr. Performance of a New Automatic Carbon Dioxide Coulometer. Microchem. J. 1977, 22, 567–573. DOI: 10.1016/0026265X(77)90128-X
(32) Gras, R.; Hua, Y.; Luong, J.; Qiao, P.; Yang, G. X.; Yang, P. Metal 3D-Printed Catalytic Jet and Flame Ionization Detection for In Situ Trace Carbon Oxides Analysis by Gas Chromatography. J. Sep. Sci. 2019, 42 (17), 2826–2834. DOI: 10.1002/jssc.201900214
(33) Luong, J.; Hua, Y.; Gras, R.; Hawryluk, M. In Situ Methanation with Flame Ionization Detection for the Determination of Carbon Dioxide in Various Matrices. Anal. Methods 2018, 10, 1275–1279. DOI: 10.1039/C8AY00079D
(34) Luong, J.; Yang, G. X.; Hua, Y.; Yang, P.; Gras, R. Gas Chromatography with In Situ Catalytic Hydrogenolysis and Flame Ionization Detection for the Direct Measurement of Formaldehyde and Acetaldehyde in Challenging Matrices. Anal. Chem. 2018, 90, 13855–13859. DOI: 10.1021/acs.analchem.8b04563
Jim Luong is with Dow Chemical Canada ULC, in Fort Saskatchewan, Alberta, Canada. Direct correspondence to: luong@dow.com

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.











