Chiral Capillary Gas Chromatography: A Highly Selective Analytical Tool
Chiral capillary gas chromatography is a highly selective and valuable tool for the analysis of enantiomers in many market areas. This selectivity allows analysts to identify marker compounds for authenticity and adulteration, determine aroma components for the development of flavors approximating natural flavors, evaluate processing and storage effects, and to control and monitor fermentation processes. Chiral selectivity can also be used to analyze for chiral metabolites, evaluate the possible biotransformation of pesticides and persistent pollutants, and investigate health promoting compounds among its many uses. The ability to resolve the enantiomers results from the use of specially prepared derivatives of cyclodextrins as stationary phases. We will discuss the utility of the chiral capillary columns and demonstrate examples of the unique selectivity of chiral gas chromatography.
One of the most important trends in chemical and biotechnology is an increasing demand for enantiomerically pure substances such as drugs, food additives, agrochemicals, and reagents for research. The most versatile, convenient, and accurate technique for the determination of the enantiomeric composition of volatile and semivolatile samples is chiral gas chromatography (GC) using capillary columns. High resolution, sensitivity and speed make chiral capillary gas chromatography ideal for the analysis of natural products and other complex mixes.
The first chiral GC separations were performed over five decades ago by Gil-Av and associates using an amino acid derivative as the stationary phase (1). Subsequent to this was the development of the ChirasilVal, which coupled L-valine tertbutyl amide into a polysiloxane based stationary phase and provided excellent resolution of derivatized D and L amino acids (2). The amino acid phases, though, were limited in their applicability, due to racemization of the chiral selector and reduced stability and selectivity at higher temperatures. It was not until the development of cyclodextrin based chiral stationary phases in the late 1980s and early 1990s that there was a rapid increase in chiral GC separations (3). This expanded the range of selectivity and stability for chiral separations.
Today, there are many derivatized cyclodextrin phases used for chiral capillary GC. Cyclodextrins (CDs) are cyclic, chiral, toroid macromolecules composed of six or more D(+)-glucose residues bonded through α(1–4) glycosidic linkages. Cyclodextrins are classified by the number of glucose units they contain; α-CDs contain six units (cyclohexylamylose), β-CDs contain seven units (cycloheptylamylose), and γ-CDs contain eight units (cyclooctylamylose).
The size and number of stereogenic centers of the cavity increases with increasing number of glucose units. α-CDs have a cavity size of 4.7 to 5.2 Å and have 30 stereogenic centers. β-CDs have a cavity size of 6.0 to 6.5 Å and have 35 stereogenic centers. γ-CDs have a cavity size of 7.5 to 8.5 Å and have 40 stereogenic centers. The mouth of the torus-shaped CD molecule has a larger circumference than the base and is linked to secondary hydroxyl groups of the C2 and C3 atoms of each glucose unit.
The primary hydroxyl groups are located at the base of the torus, on the C6 atoms. All hydroxyl groups, whether at the 2, 3, or 6 position of each residue, can be selectively modified with a derivative to impart unique selectivities. Both the architecture and chemistry of cyclodextrins contribute to enantiomer separations. The toroidal cyclodextrin structure has a hydrophilic exterior surface resulting from the 2-, 3- and 6-position hydroxyl (OH) groups. The interior cyclodextrin cavity is composed of the glucoside oxygens and methylene hydrogens, which gives it a non-polar (hydrophobic) character. Chemical interactions that lead to chiral separations occur on both the exterior and interior surfaces of the cyclodextrin toroid (4).
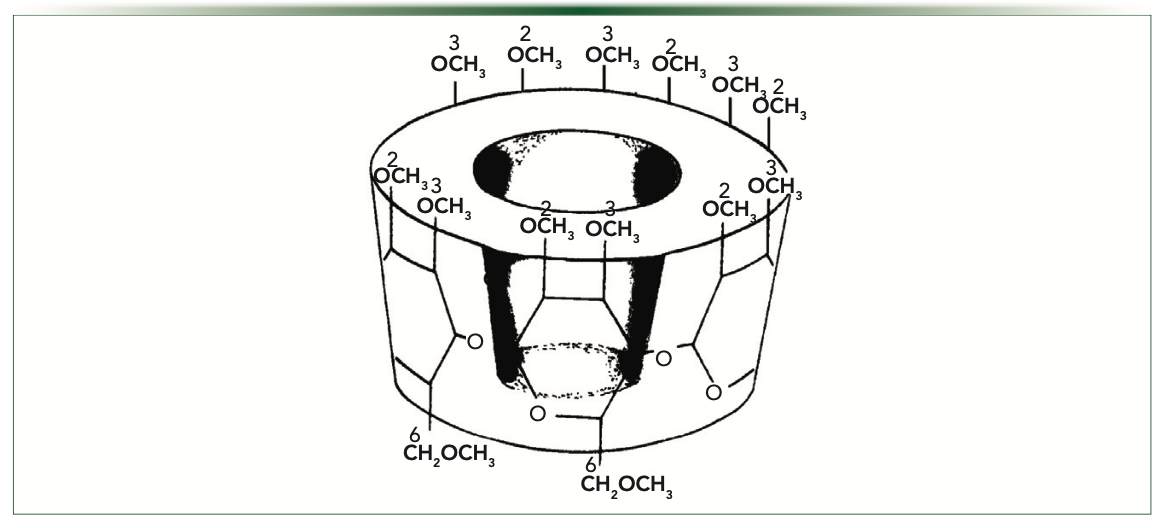
The chemistry of the derivatives of the cyclodextrins affect the selectivity and applicability of the cyclodextrin stationary phases. The most widely used derivative is the permethylated cyclodextrin. Figure 1 depicts the structure of a permethylated β-cyclodextrin. Other common derivatives include diacetyl, trifluoroacetyl, dipentyl, perpentylated, acetyl, dimethyl, dipropionyl, dipropyl, and butyryl as a few examples. The nature of the analyte as well as the polarity and position of the cyclodextrin’s derivative functional groups affects whether the separation mechanism involves an inclusion interaction or surface interactions (4). Inclusion is the process of the compound entering into the hydrophobic cyclodextrin cavity and interacting with the inner surface. Surface interactions result when the compounds interact predominantly with the functional groups on the rims of the cyclodextrins. Also, the size of the cyclodextrin (for example, α-, β- or γ-CD) can affect chiral selectivity. The majority of the derivatized cyclodextrins are typically dissolved into a nonpolar or intermediate polarity achiral stationary phase at percentages ranging from 10 to 30%.
FIGURE 1: Structure of a permethylated beta-cyclodextrin.
Cyclodextrin-based chiral stationary phases can be used to resolve many different chemical compounds. Applications have been generated for the separation of acids, amines, alcohols, diols, epoxides, esters, ethers, halogenated compounds, hydrocarbons, ketones, lactones, terpenes, and aromatic positional isomers. One area where chiral capillary GC has been widely applied is for the analysis of essential oils and flavor and fragrance compounds to determine authenticity and adulteration. The unique selectivities of the derivatized cyclodextrins and the high efficiency of capillary columns makes them ideal for such applications, as essential oils are complex matrices comprised of many compounds with a variety of functional groups. One of the most widely studied class of compounds in essential oils is the terpenoids. Pure, authentic essential oils are economically important and large quantities are produced worldwide for industries such as cosmetics, flavor, and fragrances, and for the health industry with a focus on aromatherapy and phytomedicine. The price of the oils varies, depending upon the quantity produced and based on the availability of the raw materials for the oils. Interest in using natural oil extracts continues to increase as consumers focus on natural or healthy materials compared to synthetically prepared oils. Thus, there is a need to authenticate essential oils. Biosynthetic pathways in plants typically produce a specific enantiomeric composition of terpenes and chiral capillary GC is the preferred method used to determine whether these oils have been adulterated with synthetic compounds. Authentication and adulteration can easily be detected by using chiral capillary GC to determine the composition of the marker compounds in the essential oils and by determining the enantiomeric ratios of the compounds.
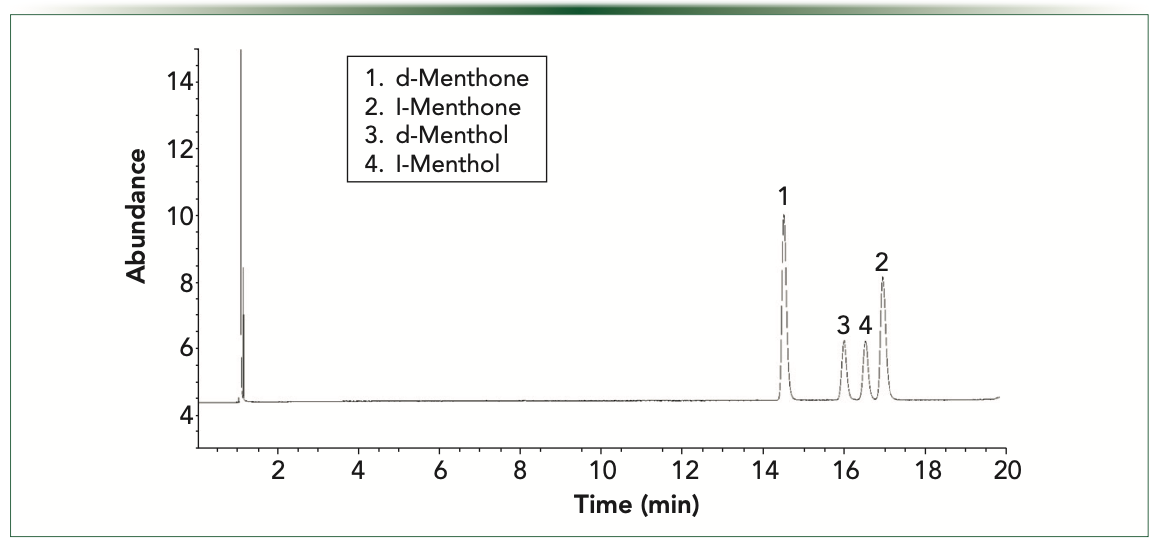
Mint oils such as peppermint and spearmint are some of the most widely used essential oils in many applications. Two major terpenes found in peppermint oils are menthol and menthone. Both are chiral compounds. Menthol has eight possible stereoisomers. Menthone has two chiral centers and four possible stereoisomers. (-)- menthone (l-menthone) is also the most naturally occurring stereoisomer. To demonstrate the differences in selectivity of two different chiral capillary columns, we analyzed samples of d and l-menthol and d and l-menthone on a Gamma Dex 225 and a ChiralDex G-TA column. Gamma Dex 225 is a 2,3-di-O-acetyl-6-0-TBDMS derivatized CD and the G-TA is 2,6-di-O- pentyl-3-trifluoroacetyl derivatized CD. Both columns were 30 m x 0.25 mm i.d., and the film thickness for the 225 column is 0.25 μm and 0.12 μm for the G-TA. Samples were analyzed under isothermal conditions as listed in Figures 2 and 3. Note the compound selectivity differences on these columns. The Gamma Dex 225 column uses an inclusion interaction with the compounds to perform the separation while the G-TA column is known to use surface interactions to perform the chiral separations.
FIGURE 2: Menthol and menthone enantiomeric separations on Gamma-Dex 225 90 °C isothermal.
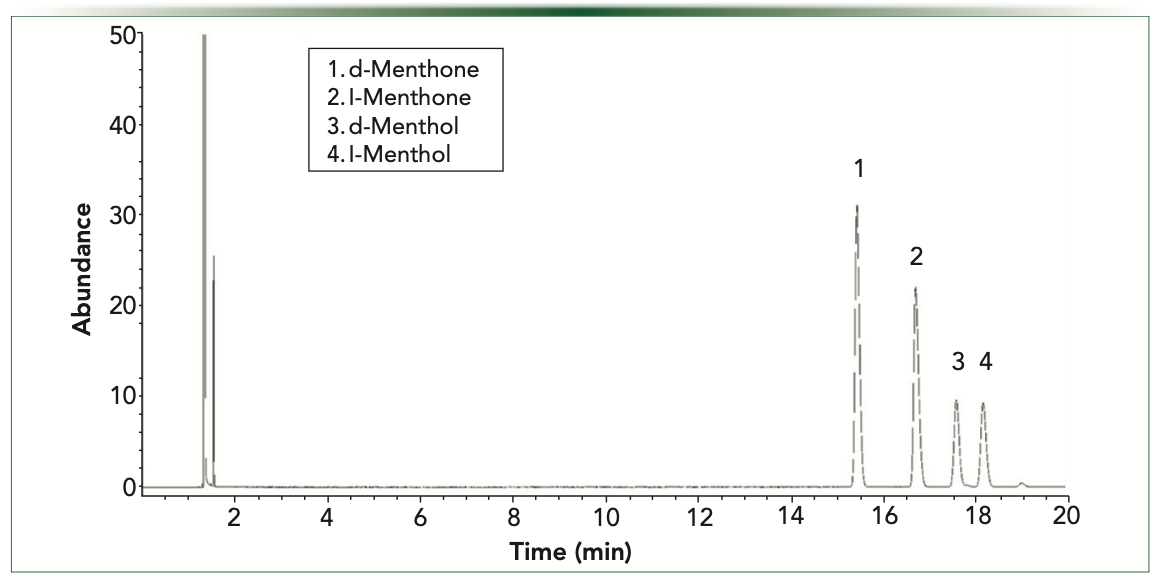
FIGURE 3: Menthol and menthone enantiomeric separations on ChiralDex G-TA 85 °C isothermal.
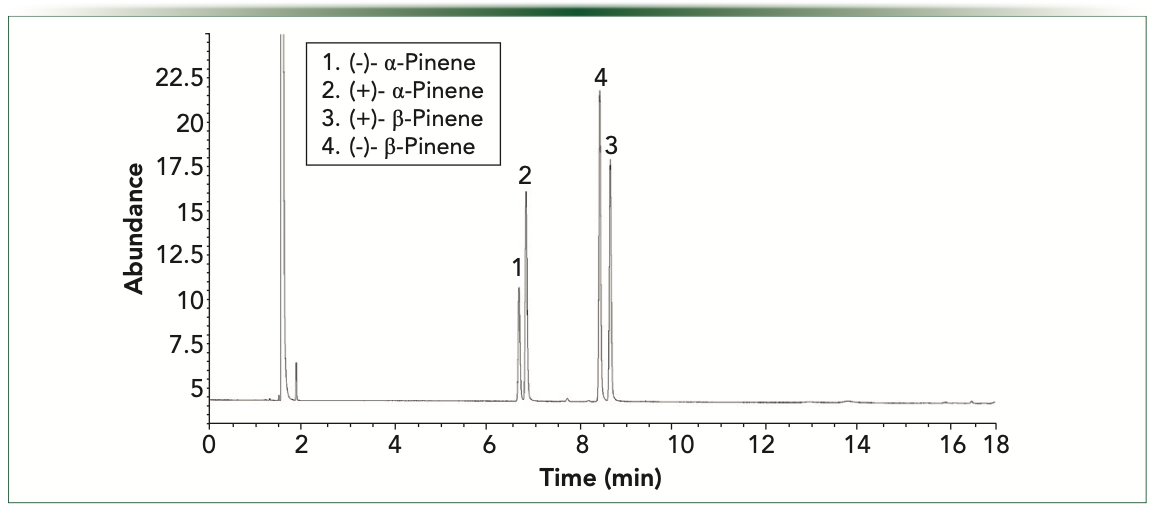
Figures 4 and 5 demonstrate the separation of alpha and betapinene enantiomers on the Beta-Dex 120 and a ChiralDex G-TA capillary columns under temperature programmed conditions, from 40 °C (1 min) at 4 °C/minute to 100 °C (1 min). These analyses demonstrate the effect of the nature and size of the derivatized cyclodextrin stationary phase on enantioselectivity, as the elution pattern of the (+) and (–) beta-pinene reverses on the ChiralDex G-TA compared to the Beta-Dex 110 column. This effect has been noted previously and can be useful in detecting and quantitating minor enantiomers in the presence of major enantiomers (5).
FIGURE 4: Alpha and beta-pinene resolution on Beta-Dex 120 temperature programmed analysis.
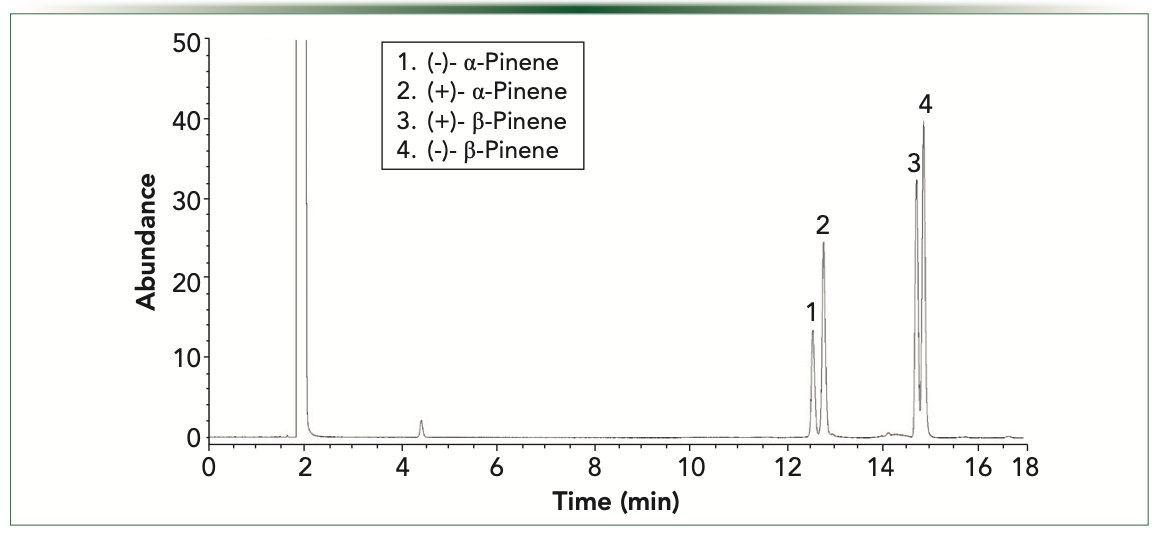
FIGURE 5: Alpha- and beta-pinene resolution on ChiralDex G-TA temperature programmed analysis.
Conclusion
Chiral capillary gas chromatography is a powerful tool for resolving enantiomers of various compounds. Differences in the size of the cyclodextrin and particularly its derivative moieties provide unique selectivities for each stationary phase. Indeed, enantioselectivities can be reversed in many cases. Facile resolution of monoterpenoid compounds found in mint oils and other natural products demonstrated the unique selectivity of the cyclodextrin-based capillary GC columns.
References
(1) Gil-Av, E.; Feibush, B.; Charles-Sigler, R. Separation of Enantiomers by Gas Liquid Chromatography with an Optically Active Stationary Phase. Tetrahedron Lett. 1966, 7 (10), 1009–1015. DOI: 10.1016/S0040-4039(00)70231-0
(2) Frank, H.; Nicholson, G. J.; Bayer, E. Rapid Gas Chromatographic Separation of Amino Acid Enantiomers with a Novel Chiral Stationary Phase. J. Chromatogr. Sci. 1977, 15, 174. DOI: 10.1093/chromsci/15.5.174
(3) König, W. A.; Lutz, S.; Mischnick-Lübbecke, P.; Brassat, B.; Wenz, G. Cyclodextrins as Chiral Stationary Phases in Capillary Gas Chromatography I. Pentylated α-cyclodextrin. J. Chromatogr. A 1998, 47, 193–197. DOI: 10.1016/0021-9673(88)90020-9
(4) Berthod, A.; Li, W.; Armstrong, D. W. Multiple Enantioselective Retention Mechanisms on Derivatized Cyclodextrin Gas Chromatographic Chiral Stationary Phases. Anal. Chem. 1992, 64 (8), 873–879. DOI: 10.1021/ac00032a009
(5) Armstrong, D. W.; Li, W.; and Pitha, J. Reversing Enantioselectivity in Capillary Gas Chromatography with Polar and Nonpolar Cyclodextrin Derivative Phases. Anal. Chem. 1990, 62 (2), 214–217. DOI: 10.1021/ac00201a023
Leonard M. Sidisky is a Senior Principal Scientist Gas Separations R&D at MilliporeSigma, in Bellefonte, Pennsylvania. Direct correspondence to: len.sidisky@milliporesigma.com

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










