Improvement of GC Sensitivity for Polar and Nonpolar PAHs by Using a Deactivated Liner
LCGC North America
This article presents the findings from an analysis conducted on sensitivity in GC on the basis of the complex group of polycyclic aromatic hydrocarbons and PAH derivatives.
Polycyclic aromatic hydrocarbons (PAHs) and their heterocyclic analogues containing nitrogen, sulfur, or oxygen represent the predominant chemical classes in the soil of former coal gasification sites, tar-oil distillation plants, and wood-preserving facilities (1). In addition to the thoroughly examined EPA PAHs and their heterocyclic analogues, further PAH derivatives with functional groups such as hydroxyl-, ketone-, carboxyl-, cyano-, nitro-, and amino-PAHs can be detected in environmental samples. These special derivatives are products of biological degradation (2–4), abiotic transformation, or incomplete combustion (5) of PAHs. To screen such samples means to analyze hundreds of polar, nonpolar, and low-volatile substances simultaneously. Although the analysis of polar compounds is sometimes difficult using gas chromatography (GC), it is a generally applied chromatographic method.
Improving the vaporization process of polar compounds in GC by derivatization: A precondition for GC determination of organic compounds is nondestructive vaporization. Hence, the measurement of polar compounds by GC could cause problems due to their weak thermal stability (6) and adsorption effects. Depending upon the chemical structure, the volatility of a compound can be improved by derivatization. However, derivatization is not only time-consuming but often incomplete as well. Thus, a quantitative analyzing after derivatization must be evaluated critically, and sometimes will not be applicable (7,8). On this account, analyzing without derivatization is preferable.
Reduction of adsorption by surface deactivation: During GC injection, the adsorption of polar and low-volatile compounds on reactive surfaces, caused by the use of nondeactivated glass wool (9,10), the reuse of a GC liner cleaned with aggressive media (11), or a strong sample matrix can lead to losses. Sometimes the problems can be solved by the adaptation of GC injector and GC oven parameters or by changing the injection solvent (3,12,13). The deactivation of the GC liner and the glass wool filling by covering their surface with an inert material is a common way to avoid these problems. For the manufactured deactivated GC liners and glass wool types, the applied deactivation techniques are trade secrets. Tests with laboratory-produced silylated GC liners showed low quality and their effects on chromatography were irreproducible. Hence, one manufactured deactivation type was used in this test as a representative.
Task of this research: On the basis of polar and low-volatile PAHs and PAH derivatives, this investigation will demonstrate the effect of deactivated GC liners and glass wool fillings on the difficult vaporization process in GC. At the same time, the study addressed the question "Which physical property of a PAH indicates a complicated vaporization process in GC?" Molecular weight, molecular structure, functional groups, and polarity influence the vapor pressure, and therefore, the adsorption behavior of compounds during GC injection.
To avoid matrix-related effects of environmental samples and to evaluate authentic PAH properties, a matrix-free standard mixture was used. The large number of possible PAHs and PAH derivatives indicates the necessity to apply a multianalyte test mixture in this study. A standard-solution mix was prepared that contained 65 nonpolar and polar PAH derivatives. Deactivated and nondeactivated GC liners were tested. After triplicate injection of the standard-solution mix, the measured peak areas were compared and the performances of the different liners were evaluated. Although effects of GC liner deactivation, different internal standards (13), and glass wool filling are described in general in literature, the adsorption properties of the tested PAH derivatives are difficult to predict in particular cases.
Materials and Methods
Chemicals: Acetonitrile (Promochem, Wesel, Germany) in high performance liquid chromatography (HPLC) gradient grade and toluene (VWR, Hamburg, Germany) in extra pure quality were used. The 16 EPA PAH standard-solution mix was ordered from Ehrenstorfer (Augsburg, Germany) containing 10 μg/mL each in acetonitrile, and the remaining PAH derivatives (Table I) were supplied by VWR (Hamburg, Germany), Sigma, Aldrich, and Fluka (Munich, Germany) at bestavailable purity. Anthracene-d10 (Promochem, Wesel Germany) was used as internal standard with a concentration of 10 μg/mL. MSTFA was obtained from Machery Nagel (Düren, Germany).
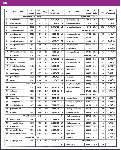
Table I
Sample preparation: The PAH standard-solution mix contained 65 polar, nonpolar, low, and highly volatile PAHs (Table I).
Each PAH was dissolved in acetonitrile to a concentration of 1 mg/mL. All PAHs were mixed and diluted to a concentration of 10 μg/mL for each derivative. A 500-μL volume of the 10-μg/mL PAH derivative standard and 500 μL of the 10-μg/mL 16 EPA PAH standard were mixed to a final concentration of 5 μg/mL of each PAH and 50 μL of the internal standard (10 μg/mL) anthracene-d10 were added. The PAH standard-solution mix is stable for days at room temperature.
To simplify the calculation of data, just one internal standard (anthracene-d10) was used in this test. Hence, processes of coating of a deactivated GC liner by matrix effects that influence its efficiency might not be recognized immediately. The use of different adapted internal standards, as described in the U.S. EPA Method TO-13a, helps to avoid this problem (14).
Preparing untreated borosilicate GC glass liners: The borosilicate glass liners (N612-1006) and the nondeactivated glass wool (item No.: 610-2354) were obtained from PerkinElmer (Shelton, Connecticut). Siltek GC liners (# 21717-214.1) and Siltek glass wool (# 21 100) were obtained from Restek (Bellefonte, Pennsylvania). Four different GC liners were tested (Table II). Nondeactivated and deactivated GC liners with and without glass wool filling were compared. Limited by the PTV injector, the GC liners are 86.2 mm long and have an internal diameter of 2 mm (volume GC liner = 0.27 mL). Before use, the borosilicate GC liners were cleaned for 2 h in nitric acid (65%) at 60 °C in an ultrasonic bath, rinsed in sequence with deionized water and acetone and dried under a nitrogen stream at room temperature.
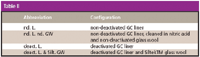
Table II
Chemical analysis: A Turbomass/Autosystem II GC–mass spectrometry (MS) system from PerkinElmer was used. The GC system was equipped with a PTV injector and a 30 m × 0.25 mm, 0.25-μm df DB5 MS GC column. A 1-μL volume of the PAH standard-solution mix was injected splitless at an injector temperature of 250 °C with normal speed. After 30 s, the split was opened.
GC oven temperature program: T1 = 80 °C; 3.5-min hold; ΔT1 = 4.8 °C/min; T2 = 320 °C, 1.5-min hold; the column-flow was 1 mL/min. All measurements of the PAH standard-solution mix were done in scan mode (50–300 amu). Only the internal standard anthracene-d10 (M+ 188) was measured in single-ion mode (Table I).
Although the GC system was equipped with a PTV injector, the samples were flush injected at 250 °C in a PTV GC liner. An injection at a low temperature as recommended would have led to an increased risk of adsorption effects for the injected analytes. Problems of overcharging were avoided by a small injection volume of 1 μL. Not absolute but relative data for deactivated and nondeactivated GC liner equipments will be presented in this research. Hence, all remaining negative effects, based upon the small GC liner volume, were eliminated.
Data evaluation: For data processing, the peak areas of all PAHs were calculated. Therefore, the total ion chromatograms (m/z 50–300) were transferred to single-ion chromatograms depending upon the corresponding quantification masses (Table I). Finally, the relevant peak areas (APAH) were evaluated, standardized (1) (APAH) by the internal standard (ISTD), and averaged (2) with respect to the triplicate measurement (APAH).
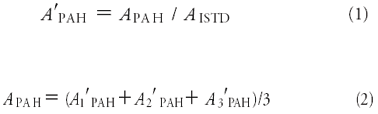
The influence of glass wool on the reproducibility for all PAHs was accessed by the mean value (VC) of the coefficients of variation (VC). Therefore, the coefficient of variation (VC) of each PAH and triplicate measurement was calculated and, finally averaged (VC) for each GC liner type.
Considering a comparison of performance with respect to sensitivity, the standardized, averaged peak areas for the deactivated GC liner equipment were divided by the standardized, averaged peak areas of the nondeactivated GC liner equipment.
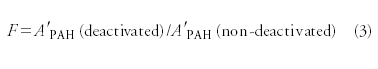
Results and Discussion
Factors of influence on reproducibility: Before investigating the influences of GC liner deactivation on sensitivity, the influence of glass wool filling and deactivation on reproducibility was researched and expressed by the coefficient of variation for the triplicate measurement. These coefficients of variation were calculated for each PAH and all GC liner equipment, as described in data evaluation. Comparing the mean coefficients of variation over all PAHs and PAH derivatives for the different GC liners (Table III), the improvement of reproducibility by glass wool filling is obvious. But the mere deactivation of the GC liner without glass wool filling does not lead to a better reproducibility. The sole combination of glass wool filling and equipment deactivation results in a remarkable enhancement.

Table III
Grob (10) described the reason for this effect. In an empty GC liner, some droplets of an injected liquid sample fall to the bottom of the injector, resulting in the dissolved analyte being practically lost for the analysis. Glass wool prevents this. Sometimes in GC, the use of glass wool is made responsible for irreversible adsorptions and irreproducible results. The results of this investigation do not provide any reference with respect to reproductivity. The use of glass wool filling enhances reproducibility for both polar and nonpolar PAHs.
Recoveries Observed in the GC Injection-System
This investigation examines the influence of the GC liner deactivation on the wide range of polar and nonpolar PAHs and searches for an indicator, which points to a difficult vaporization process. Therefore, the standard-solution mix of PAHs was triplicate injected into a GC system equipped with either a deactivated or a nondeactivated GC liner. With respect to the previous results, all liners were equipped with glass wool. To examine the influence of GC liner deactivation on sensitivity, other parameters such as the GC and MS parameters were not changed. Hence, the PAH signals were just influenced by the vaporization process in the GC liner. A loss of material results in a decrease of the mass spectrometer signals.
The peak area of each PAH was calculated and then related as described in the data evaluation section of this article. The calculated quotients QPAH are compound-specific and express the change in sensitivity caused by the deactivation of the GC liner equipment. Values of F that are larger than 1 mean a gain of sensitivity, and values of F that are smaller than 1 mean a loss by the use of the deactivated GC liner equipment.
To get an idea of the physical properties of a compound that influences the evaporation process in the GC liner, this process should be outlined. When a liquid sample is injected rapidly into a hot GC injector equipped with a GC liner that is filled with glass wool, the solvent does not vaporize immediately. First of all, the solvent coats the glass wool (10) and then, due to the injector temperature (250 °C), the solvent and the dissolved compound vaporize. The boiling point of a compound, its behavior in the solvent, as well as adsorption processes on the GC liners surface predominantly influences its volatility. The distribution of a compound between the gas phase and a solvent can be described by Henry's Law constant (13). PAHs with a low Henry's Law constant are particularly at risk to undergo irreversible adsorption on the surface of a GC liner. The deactivation of the GC liner equipment would be most effective for these PAHs and PAH derivatives. Because the Henry's Law constants were not available for helium–acetonitrile distribution, the constants for air–water distribution were used.
Figure 1a shows the quotients QPAH arranged according to their corresponding Henry's Law constants. The horizontal line at F = 1 marks the boundary between loss and gain of sensitivity by deactivation. Most of the quotients are above or near to this line. This means the deactivation of the GC liner equipment does not lead to a loss of PAHs. Those PAHs that show a gain of sensitivity above 20% (F > 1.2) are marked by their serial numbers (Table I).
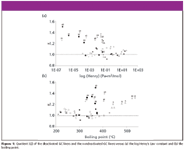
Figure 1
As presumed, the use of a deactivated GC liner leads to gain of sensitivity for PAHs that have a low Henry's Law constant (< 0.0001 Pa*m3 *mol–1 ). This group of PAHs includes hydroxyl-, ketone- and N-hetero-PAHs. Nevertheless, there are PAHs that have high Henry's Law constants and show an increased sensitivity, too. All of them are members of the EPA PAHs and have a high boiling point.
Figure 1b shows the quotients QPAH arranged according to their corresponding boiling points. Only those PAHs whose boiling point is above 440 °C show an increasing sensitivity above 20%. The reason is that the injector temperature (250 °C) is too low to enable a fast vaporization process for those high boiling compounds. Hence, their dwell time on the liner surface is increased and the compounds run the risk of irreversible adsorption. A higher injector temperature will level this effect. For this reason, predominantly PAHs with a low Henry's Law constant are positively influenced by deactivated GC liner equipment.
Acknowledgment
The authors would like to thank BMBF (Federal Ministry of Education and Research, Germany) for their financial support.
Dietmar Meyer, Hella Dressler, and Wolfgang Ruck
University of Lüneburg, Institute of Ecology and Environmental chemistry, Scharnhorstraße 1, 21335 Lüneburg
Please direct correspondence to Dietmar Meyer at dmeyer@uni-lueneburg.de
References
(1) G. Collin and M. Zander, Ullmans Enzyklopädie der Technischen Chemie, (Verlag Chemie, Weinheim, 1985), pp. 411–446.
(2) K. Broholm, B. Nilsson, R.C. Sidle, and E. Arvin, J. Contam. Hydrol. 41, 239–260 (2000).
(3) I.M. Cozzarelli, M.J. Baedecker, R.P. Eganhouse, and D.F. Goerlitz, Geochem. Cosmochim. Acta. 58, 863–877 (1994).
(4) S. Fetzner, Appl. Microbiol. Biotechnol. 49, 237–250 (1998).
(5) L. Moeller, I. Lax, and L.C. Eriksson, Environ. Health 101 (Suppl. 3), 309–315 (1993).
(6) G. Zink and K.E. Lorber, Chemosphere 31(9), 4077–4084 (1995).
(7) D. Rood, A Practical Guide to the Care, Maintenance and Troubleshooting of Capillary Gas Chromatographic Systems (Hüthig Buch Verlag GmbH, Heidelberg, 1995).
(8) K. Blau, and J. Halket, Handbook of Derivatives for Chromatography (John Wiley & Sons, Chichester, 1997).
(9) K. Grob, Classical Split and Splitless Injection in Capillary GC (Hüthig Buch Verlag GmbH, Heidelberg, 1986), p. 120.
(10) S. Bieri, P. Christen, M. Biedermann, and K. Grob, Anal. Chem. 76, 1696–1701 (2004).
(11) J. Drozd, Chemical Derivatization in Gas Chromatography (Elsevier Scientific Publishing Company, Amsterdam, 1981).
(13) L.M. Blumberg and M.S. Klee, Anal. Chem. 72, 4080–4089 (2000).
(14) Methods for Organic Chemical Analysis of Municipal and Industrial Wastewater 1999, U.S. Environmental Protection Agency, Environmental Monitoring and Support Laboratory, Cincinnati, OH 45268, EPA Compendium Method TO-13a, 625/R-96-010b, Jan. 1999.

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.