Application of SEC-MALS in Paint Manufacturing
LCGC North America
The authors explore how the development of improved light- scattering detection, ethodology, and data interpretation has allowed detailed characterization of polymers used in the paint industry.
SEC in its conventional mode is typically performed with refractive index (RI) detection, and to determine molar mass it is necessary to find the relationship between the elution volume and molar mass of eluted molecules. This calibration procedure typically is carried out by the measurement of a series of standards of known molar mass and narrow molar mass distribution. However, such standards are available only for a very limited number of polymers. In the area of synthetic polymers, narrow polystyrene standards are widely used for establishing the SEC calibration. The obtained calibration relation can be transferred to a calibration of another polymer using a so-called universal calibration procedure that requires knowledge of constants of the Mark–Houwink equation. It is necessary to point out that neither suitable calibration standards nor the constants of the Mark–Houwink equation are available for most of the polymer binders used in paint manufacturing. The column calibration by polystyrene standards is employed primarily for the characterization of polymer binders in paint research and development and manufacturing. The obtained results can be used solely for comparison of molar mass distribution of similar samples, but it is necessary to emphasize that they are often markedly far from the true values with no ability to reveal branching or non-SEC separation. Unfortunately, many SEC users are not aware of this fact and the results of conventional SEC often are misinterpreted.
The calibration problem can be solved most effectively by using multiangle light scattering (MALS) detection capable of on-line measurement of molar mass of molecules eluted from SEC columns. SEC–MALS is actually the only routine analytical technique that can yield true molar mass information for various polymer binders used in paint manufacturing. The application of MALS detection not only eliminates the need for column calibration, but also brings other advantages. The SEC–MALS method is not only independent of the calibration standards, but it is also significantly less sensitive to flow-rate variations, temperature variations, injected mass, SEC column performance, and non-SEC separation mechanisms. Consequently, a typical feature of SEC–MALS is very good long-term repeatability, which makes it an ideal tool for production quality control. In addition, data generated in various laboratories using different SEC–MALS setups are very reproducible. SEC–MALS also can provide additional information about the polymers, such as the root mean square (RMS) radius (also called radius of gyration), detection and characterization of branching, or detection of even trace amounts of fractions with extremely high molar mass. By plotting the molar mass against the elution volume, SEC–MALS can reveal non-SEC separation mechanisms and, thus, help to find appropriate separation conditions. It is also very important that MALS detection is readily adaptable, that is, it can be added easily to any current SEC system without the need for any special arrangements.
There is a general demand for volatile organic compound (VOC) reduction, which can be achieved by decreasing the solvent content. However, the reduced solvent content results in an undesirable increase of paint viscosity. The problem can be solved partly by the application of branched or hyperbranched polymers that can be well characterized by SEC–MALS. Branching influences not only the solution viscosity, but also other important polymer properties including mechanical properties and chemical resistance. Both molar mass and molecular size must be measured to get branching characterization. Unlike classical light-scattering measurement in a batch mode, the combination of SEC–MALS yields molar masses and sizes for almost monodisperse polymer fractions separated by SEC, and the polymer conformation can be revealed from the relation between the RMS radius and molar mass. The sensitivity of state-of-the-art MALS detection allows the determination of molar mass as low as a few hundreds. However, the RMS radius can be measured from about 10 nm, which means for molecules having a molar mass of about 105 g/mol and more. The limitation comes from the fact that molar mass is determined from the intensity of scattered light extrapolated to zero angle, whereas the RMS radius is determined from the slope of the angular variation of scattered-light intensity. Small polymer molecules scatter light with identical intensity in all directions and their RMS radius cannot be determined. Because many polymer molecules used in paint manufacturing have a molar mass below 105 g/mol, another type of detection must be used for the determination of size. On-line viscometry can be used together with the SEC–MALS setup to measure molar mass and size simultaneously. The size is expressed as the intrinsic viscosity and the experimental data allow determination of the so-called Mark–Houwink plot. The slope of the plot (an exponent of the Mark–Houwink equation) for linear polymers in thermodynamically good solvents is typically around 0.7 and decreases with increasing degree of branching. The method does not require data for the corresponding linear polymer, which is very important because many branched polymer binders cannot be prepared in a purely linear conformation.
Environmental regulations result in growing production of waterborne paints. These paints often are based upon polymers containing polar functional groups that increase the probability of strong enthalpic interactions with SEC column packings. SEC itself generally cannot reveal these interactions, but the obtained results often suffer poor reproducibility. The results generated by MALS detection are independent of SEC separation and, thus, superior reproducibility over conventional SEC is achieved. In addition, the interaction of the polymer with the column packing is revealed readily from the molar mass versus elution volume plot.
Experimental
The SEC–MALS setup consisted of a Waters Alliance 2695 Separations Module (Waters, Milford, Massachusetts), a miniDAWN photometer (Wyatt Technology Corporation, Santa Barbara, California), a ViscoStar viscometer (Wyatt Technology Corporation), and an RI 2414 detector (Waters). Two PLgel Mixed-C or Mixed-E columns (Varian, Palo Alto, California) were used for SEC measurements. Tetrahydrofuran at a flow rate of 1 mL/min was used as a mobile phase. Samples were injected as solutions in tetrahydrofuran in a 100-μL volume and various concentrations in the range of about 0.2–2% (w/v). ASTRA software (Wyatt Technology) was used for data collection and processing. Polymer binders were either commercially available or prepared in the author's laboratory (SYNPO, Pardubice, Czech Republic). Specific refractive index increments of the analyzed binders, which are needed to determined molar mass from the intensity of scattered light, were calculated by ASTRA software from the injected mass and response obtained from the RI detector.
Results and Discussion
Several applications of SEC–MALS for the characterization of various paint binders are demonstrated in this article to demonstrate how SEC–MALS can provide essential information in the paint research and manufacturing process.
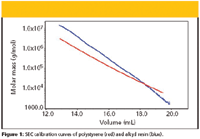
Figure 1
Alkyd resins are polymers used almost exclusively in paint manufacturing. Alkyd resins can differ not only in molar mass distribution, but also in the degree of branching. The characterization of branching is complicated by the fact that it is impossibile to prepare linear alkyd of the same composition. Alkyds are also a good example of polymers for which neither suitable calibration standards nor parameters of universal calibration are available. Alkyds represent a traditional paint binder that has been replaced in many application areas by other polymers yielding better paint properties. However, the great advantage of alkyds can be found in the fact that they are made partly from natural oils, that is, renewable raw materials and, thus, continuous interest in these polymers can be expected. Figure 1 compares molar mass versus elution volume plots (that is, SEC calibration curves) of alkyd resin and polystyrene. Both plots intersect, and toward lower elution volumes, the plot of alkyd shifts markedly to higher molar masses, which is given by the branched (that is, more compact) structure of alkyd molecules. Possible errors acquired by conventional SEC with polystyrene calibration are shown in Table I.

Table I: Comparison of apparent molar mass averages of alkyd resins determined by conventional SEC with polystyrene calibration with the true values from SEC-MALS
Figure 2 depicts the RMS radius versus elution volume plots of three samples taken from the batch during the synthesis of alkyd resin. The plot for the lowest conversion sample decreases evenly with the increasing elution volume as can be expected for the separation by the mechanism of size exclusion, while the plots of the other two samples rise at higher elution volumes. This abnormal SEC elution behavior is caused by entanglement (5) of large, highly branched molecules in the column packing and their delayed elution together with smaller molecules separated by pure size exclusion. As a consequence of the delayed elution, the slices at higher elution volumes are contaminated by small amounts of high molar mass fractions, and MALS detection measures the weight-average molar masses and the z-average RMS radii. The z-averages are more sensitive to the high molar mass fractions than the weight-averages, which is the explanation of the abnormal upswing.
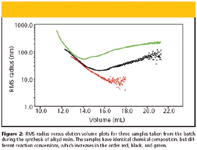
Figure 2
The Mark–Houwink plot of an alkyd resin is shown in Figure 3. The Mark–Houwink plot was obtained by completing the SEC–MALS setup with an on-line viscometer. The slope of the plot of 0.34 is well below the values for linear random coils in thermodynamically good solvents (around 0.7) and gives evidence about the presence of branched molecules.
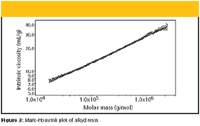
Figure 3
Paint binders often consist of oligomeric compounds that can increase molar mass by further reactions during paint application. An example of the SEC-MALS analysis of an oligomeric paint binder is shown in Figure 4. This example proves the ability of modern light-scattering detection to measure molar masses down to a few hundreds. It is worth mentioning that a part of the scientific community still considers light scattering capable of characterizing only polymers having molar mass above 10,000 g/mol, which is certainly not true, as seen from Figure 4.
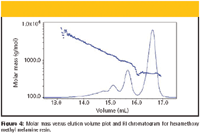
Figure 4
Figure 5 presents molar mass versus elution volume plots of two cationic polymers containing neutralized amine groups. Such polymers typically interact with the column packing strongly, which results in poor separation according to the hydrodynamic size. SEC–MALS can reveal these interactions readily and, in addition, provide at least correct weight-average molar mass (Mw ), which is determined by the MALS detector independently of separation. The ability to measure the molar mass as a function of elution volume can be further used for the modification of separation conditions to eliminate non-SEC effects.
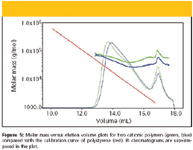
Figure 5
Conclusions
SEC–MALS can be used routinely for the analysis of various paint binders. The method is able to provide correct molar mass distribution, including samples of very low molar mass. In addition, it can reveal undesirable non-SEC mechanisms and yield at least correct Mw , even in the case of poor SEC resolution. A very important feature of SEC–MALS is its ability to characterize branching, which can be enhanced further by the addition of an on-line viscometer.
Stepan Podzimek and Daniela Vareckova
SYNPO, 532 07 Pardubice, Czech Republic
Please direct correspondence to Stepan Podzimek at stepan.podzimek@synpo.cz
References
(1) C.-Y. Kuo, T. Provder, and A.F. Kah, Organic Coatings, Science and Technology, vol. 6, G.D. Parfitt and A.V. Patsis, Eds. (Marcel Dekker, Inc., New York, 1984), pp. 101–124.
(2) L. Mandik, Progr. Organic Coatings 5, 131–198 (1977).
(3) P.J. Wyatt, Analytica Chim. Acta 272, 1–40 (1993).
(4) S. Podzimek, J. Appl. Polym. Sci. 54, 91–103 (1994).
(5) S. Podzimek, T. Vlcek, and C. Johann, J. Appl. Polym. Sci. 81, 1588–1594 (2001).

Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Determining the Serum Proteomic Profile in Migraine Patients with LC–MS
April 17th 2025Researchers used liquid chromatography–mass spectrometry (LC–MS) in their proteomic analysis to compare the serum proteome of migraine patients with healthy controls and to identify differentially expressed proteins as potential migraine biomarkers.