Improved Characterization of Malodours in Recycled Plastics
Thermal desorption (TD) with two-dimensional gas chromatography–time-of-flight mass spectrometry (GC×GC–TOF-MS) can be used for comprehensive characterization of the volatile organic compounds emitted by recycled plastics. This article presents a method that overcomes the limitations of current malodour detection techniques and is easy to translate to routine quality control (QC) screening.
A push towards a circular economy, in which materials are reused or recycled for as long as possible, has led to plastics manufacturers being urged to produce or use more post‑consumer recycled (PCR) plastics, especially for food and beverage packaging. However, PCR plastics require more rigorous quality control (QC) measures than new plastics to ensure that they will not produce volatile emissions that could be considered harmful or have a negative impact on the packaged product (for example, malodours). Unfortunately, existing methods for the detection of odours from plastics have several limitations.
A human sensory panel is a sensitive approach, but it is also subjective, time‑consuming, and requires skilled individuals. In addition, it is restricted to sensory information: no chemical identities are provided, so sensory panels cannot identify a possible source or clean-up process to eliminate
the malodour.
The electronic nose (eNose) is faster and simpler to use as it is a handheld device with sensor technology. However, the technique is not specific, meaning that samples that fail QC testing must undergo further analytical investigation.
Gas chromatography coupled with mass spectrometry (GC–MS) can provide a more quantitative approach, but it may struggle to fully resolve all the volatile organic compounds (VOCs). Typically, the odour profiles are dominated by aliphatics from the polymer itself, which easily mask the trace-level odorants (for example, oxygenated species). Traditional quadrupole MS must operate in scan mode to find these nontarget components, but this limits the sensitivity of the instrument. Additionally, common sample introduction techniques, such as headspace injection and solid-phase microextraction (SPME), may lack the necessary sensitivity to capture the trace odorants.
With these approaches, it is often not possible to identify the precise compounds responsible for high odour in recycled plastics, which means that the recycling process cannot be improved and QC failures continue to occur. Trace odours can cause the end users’ products to fail QC further down the production chain, which passes further cost onto the customers and can cause losses in returned product or compensation claims.
Thermal desorption coupled with comprehensive two-dimensional gas chromatography and time-of-flight mass spectrometry (TD–GC×GC–TOF-MS) can address these challenges by providing high sensitivity and improved separation of the odour profiles prior to confident identification of the individual analytes.
In this article, we demonstrate how TD–GC×GC–TOF-MS can provide confident characterization of complex odour profiles from recycled plastics for fast and simple identification of the compounds causing QC failures. Once the key odorants are known, methods can be easily translated to TD–GC×GC–flame ionization detection (FID) for routine screening in QC laboratories at production sites.
Experimental
Samples: An empty TD tube (Markes International) was filled with 0.3 g of plastic pellets. TD: Instrument: TD100-xr (Markes International); focusing trap: Materials Emissions (Markes International); trap low: -20 °C; desorption temperature: 100 °C. GC×GC: Insight flow modulator (SepSolve Analytical); modulation period (PM) = 2.5 s. TOF-MS system: BenchTOF2 time-of-flight mass spectrometer (SepSolve Analytical); mass range: m/z 30–600. Software: ChromSpace software (SepSolve Analytical) for full instrument control and data processing, with chemometric comparisons by ChromCompare+ (SepSolve Analytical).
Method: First, the VOCs were sampled using direct desorption in which a small number of plastic pellets were placed directly into an empty TD tube, which was heated to release the VOCs. Optimal sensitivity was achieved by preconcentrating the analytes on an electrically cooled focusing trap before they were sent to the GC system in a narrow band of vapour. Some TD instruments can enable automated analysis of up to 100 tubes and operate solvent‑free and cryogen-free, making them ideal for high-throughput screening of plastics.
Next, GC×GC using a consumable-free flow modulator provided the separation necessary to resolve such complex odour profiles, to obtain clean spectra and confident identification of analytes by the TOF mass spectrometer. TOF‑MS provided the acquisition speeds necessary to cope with the narrow peak widths generated by GC×GC, as well as improved sensitivity and selectivity compared to single quadrupole MS, making it the ideal technique for untargeted “discovery” applications.
Finally, software compared the resulting chromatograms, enabling the important differences between high- and low-odour plastics to be identified. This automated, untargeted workflow used all the raw data to minimize the risk of missing any compounds of importance.
Results and Discussion
In one-dimensional (1D) GC separations, the trace odorants in plastics are often masked by the high-loading aliphatics and may be overlooked entirely. Figure 1 provides the TD–GC×GC–TOF-MS chromatogram for the direct desorption of plastic pellets. The aliphatics were retained longer in the second‑dimension column, thus eluting in a band of intense peaks along the top of the colour plot (as annotated in Figure 1). On the other hand, the odour-active compounds (such as oxygenated species) eluted earlier in the second dimension, separating these compounds from the aliphatics.
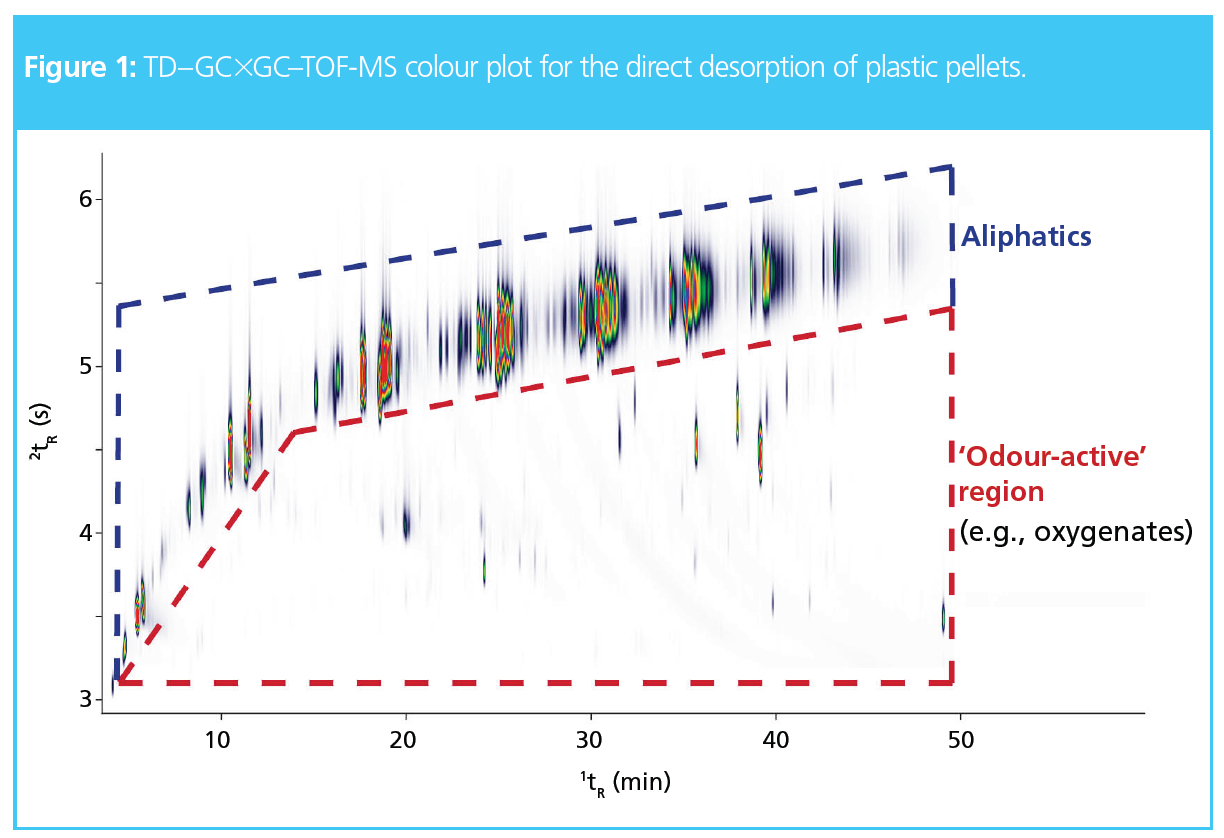
For example, Figure 2 shows a zoomed region of the chromatogram in which a trace peak is physically separated from the high‑loading aliphatics and could be confidently identified as nonanal, which is known to impart a waxy, aldehydic odour (1).
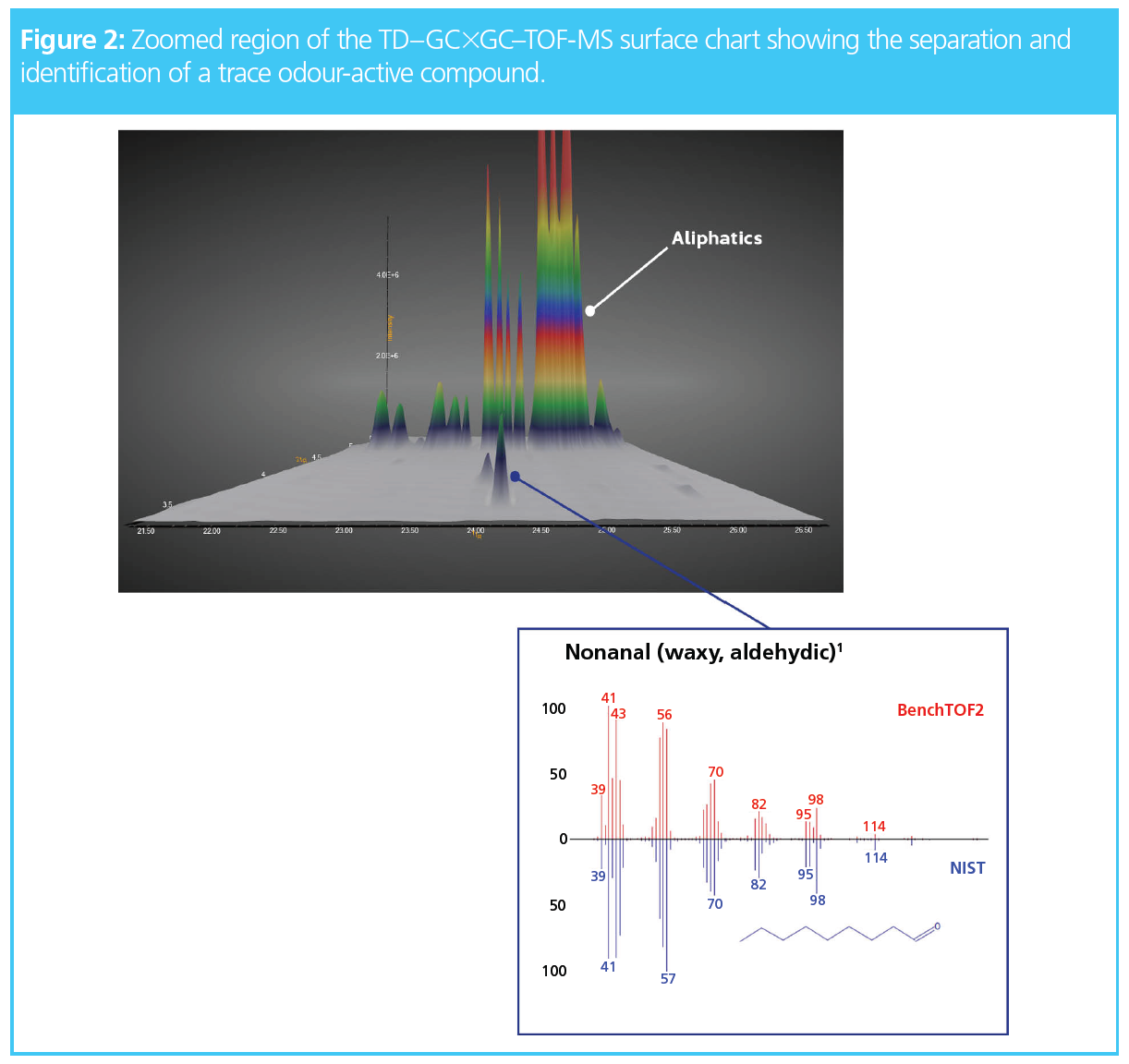
It is important to note that the rapid secondary separations in GC×GC frequently result in peak widths less than 100 ms, so detector speeds of 100 Hz are essential to maintain at least 10 datapoints across a peak. A TOF-MS system is inherently well-suited to handling such narrow GC peaks, since they are dispersive (not scanning) instruments, and so effectively monitor all masses at once with fast acquisition rates. The ability to record full-range mass spectral information to extremely high densities enables TOF-MS to handle the narrowest peaks encountered in well‑optimized GC×GC couplings.
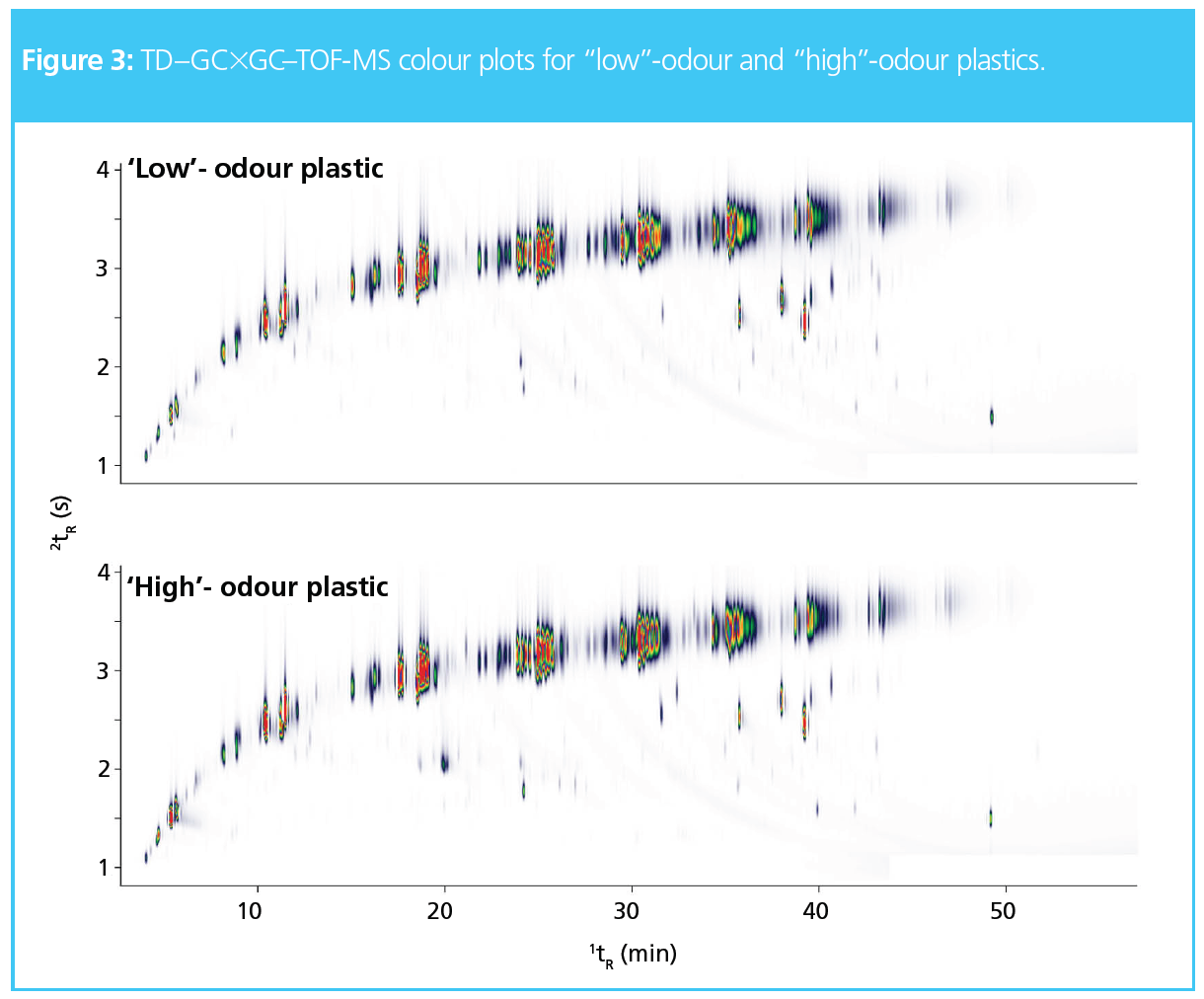
To prove the performance of TD–GC×GC–TOF-MS to identify differences between plastics that have passed and failed QC tests, a selection of “high”-odour and “low”-odour plastics were analyzed. It is clear from the example chromatograms in Figure 3 that there are additional peaks in the odour-active region of the high-odour plastic. However, for such high‑throughput applications, automated comparison of samples is essential to deliver fast results.
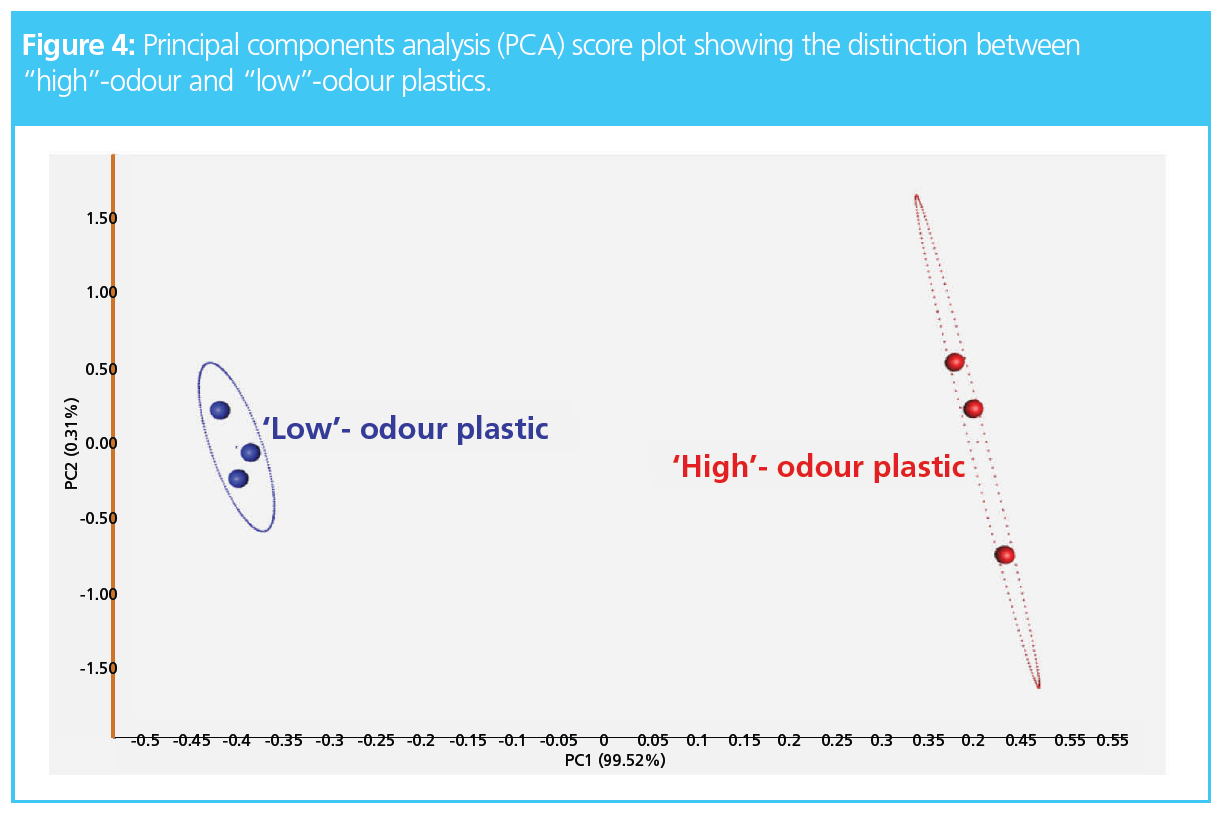
Here, software was used to automatically compare all the raw TD–GC×GC–TOF‑MS data for three high-odour plastics and three low-odour plastics to identify the key odorants responsible. The resulting principal components analysis (PCA) score plot (Figure 4) shows the clustering of the low- and high-odour plastics, indicating there are compositional differences between their odour profiles.
The software could identify the components found in increased amounts in the high-odour pellets. For example, Figure 5 shows one of the top differentiators, which was identified as n-octanol. It was found in increased abundance in the high-odour pellets and is known to impart a waxy odour (1).
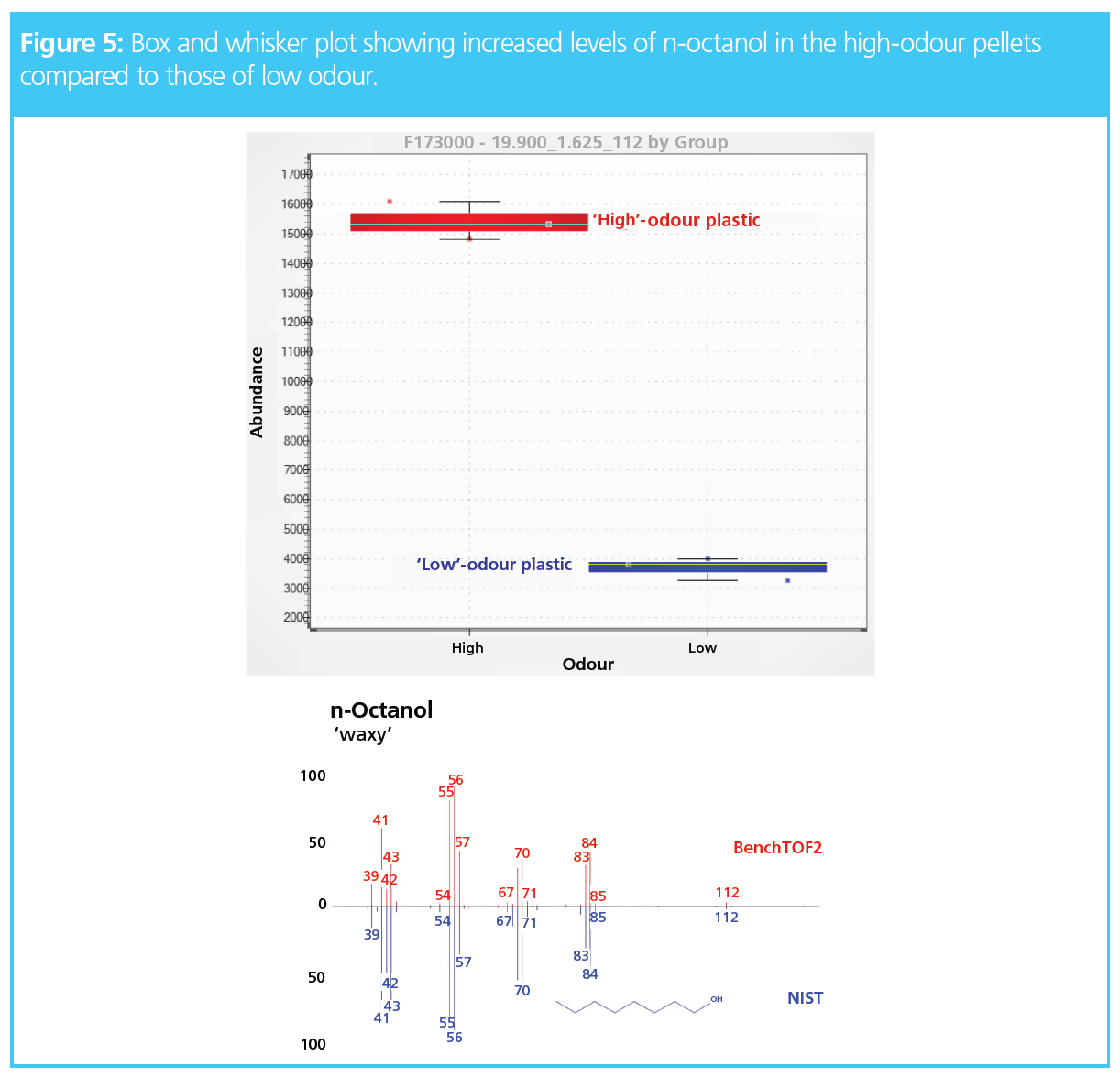
Once the key odorants were identified, a class prediction model was developed in the software to instantly classify future unknown samples. This is a key aspect for QC labs, where instrumentation and workflows must be as simple and cost-effective as possible. With this in mind, TD–GC×GC methods can also be easily translated to FID, for routine use in quality control laboratories.
Conclusions
This study has shown that TD–GC×GC–TOF-MS provided automated cryogen-free sampling of VOCs from plastics using direct desorption in TD tubes. Detection of trace odorants was improved using TD preconcentration and high-sensitivity TOF-MS, while flow-modulated GC×GC provided enhanced separation and discovery of odorants that could not be identified using 1D GC–MS. Reference-quality spectra generated by TOF-MS enabled key odorants to be confidently identified. The workflow was streamlined using a single software platform for full instrument control and data processing, with chemometrics to automatically find differences between complex chromatograms. Flexible configurations with simple translation from TD–GC×GC–TOF‑MS to TD–GC×GC–FID mean that the setup could be used in QC laboratories.
References
- The Good Scents Company Information System (search facility), www.thegoodscentscompany.com/search2.html (accessed 2 August 2021).
Laura McGregor received an M.Chem. in chemistry from the University of St Andrews, UK, followed by an M.Sc. in forensic science at the University of Strathclyde, UK. Her Ph.D. in environmental forensics, also at the University of Strathclyde, focused on the chemical fingerprinting of environmental contamination using advanced techniques such as GC×GC−TOF-MS. In her current role at SepSolve Analytical, she specializes in the application of GC×GC and TOF-MS to challenging applications.
Elinor Hughes obtained her B.Sc. in chemistry and Ph.D. in organic chemistry at Bangor University, UK. After working for a chemical manufacturing company for three years, she moved to the Royal Society of Chemistry where she worked in journals publishing for six years and on Chemistry World magazine for four years. Her current role is technical copywriter at Markes International.
E-mail: hello@sepsolve.com
Website: www.sepsolve.com

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.


















