Effectiveness of High-Resolution Ion Mobility for Complex Analyses
Ion mobility is a structurally selective separation strategy that is now routinely integrated with mass spectrometry (IM-MS) to improve peak capacity while simultaneously aiding in the separation of isobaric signals such as those encountered in complex chemical samples. Structures for lossless ion manipulation (SLIM) technology provides a modular architecture for developing tailored ion processing systems, including ion mobility spectrometers. This article evaluates the separation capabilities of a SLIM‑based high-resolution ion mobility spectrometer (HRIM) incorporating an extended (~13 m) separation path length design in order to determine benefits for scientists working with complex and challenging analyte classes such as glycans, peptides, and lipids.
While conventional ion mobility (IM) does not perform at the same level of selectivity and resolution as liquid and gas chromatography (LC and GC), IM separations are significantly faster than LC and GC (100s of ms vs. minutes), and IM can be coupled online with chromatography techniques or mass spectrometry (MS) imaging to further increase peak capacity (1).
Concomitant with chemical separations, IM provides an additional molecular measurement in the form of gas-phase collision cross sections (CCSs), which can provide further structural insight and an additional molecular descriptor useful for compound identifications (2). Many of the technical hurdles associated with integrating IM with MS, such as poor sensitivity and limited throughput, have largely been addressed in the latest generation technologies. However, one contemporary challenge of IM has been the limited resolution imposed by the conventionally short, sub-metre path lengths used.
High-resolution ion mobility-mass spectrometry (HRIM-MS) based on structures for lossless ion manipulation (SLIM) technology has emerged as a promising separation strategy due to the ease at which this architecture can be scaled up to longer separation path lengths. HRIM-MS uses printed circuit boards (PCBs) held in a chamber maintained at a constant, reduced pressure (2–4 Torr). The PCBs have a series of radio frequency (RF), direct current (DC), and travelling wave electrodes printed on them that provide an ion conduit through which the analytes traverse along a separation path. The electric fields that propel the ions also prevent them from striking surfaces while moving, therefore preventing any losses along their way. Depending upon the speed of the travelling wave, ions either “surf” and are not separated (as in a total ion transmission mode), or they undergo enough collisions with the gas molecules present that they roll over the travelling wave peaks and IM separation occurs (3,4).
In HRIM, as with other IM technologies, an ion’s migration time is determined by its gas-phase size-to-charge ratio, which is representative of the analyte size and shape. As ions are driven along the separation path, collision with an inert buffer gas slows them down to a degree proportional to their size. The length of the ion separation path is crucial to achieving the high degree of separation required to gain adequate resolution of compounds with challenging structural diversity. In this HRIM arrangement, the ion path is 13 m (roughly half the length of a tennis court), allowing for high resolution separation power in a compact design (Figure 1).
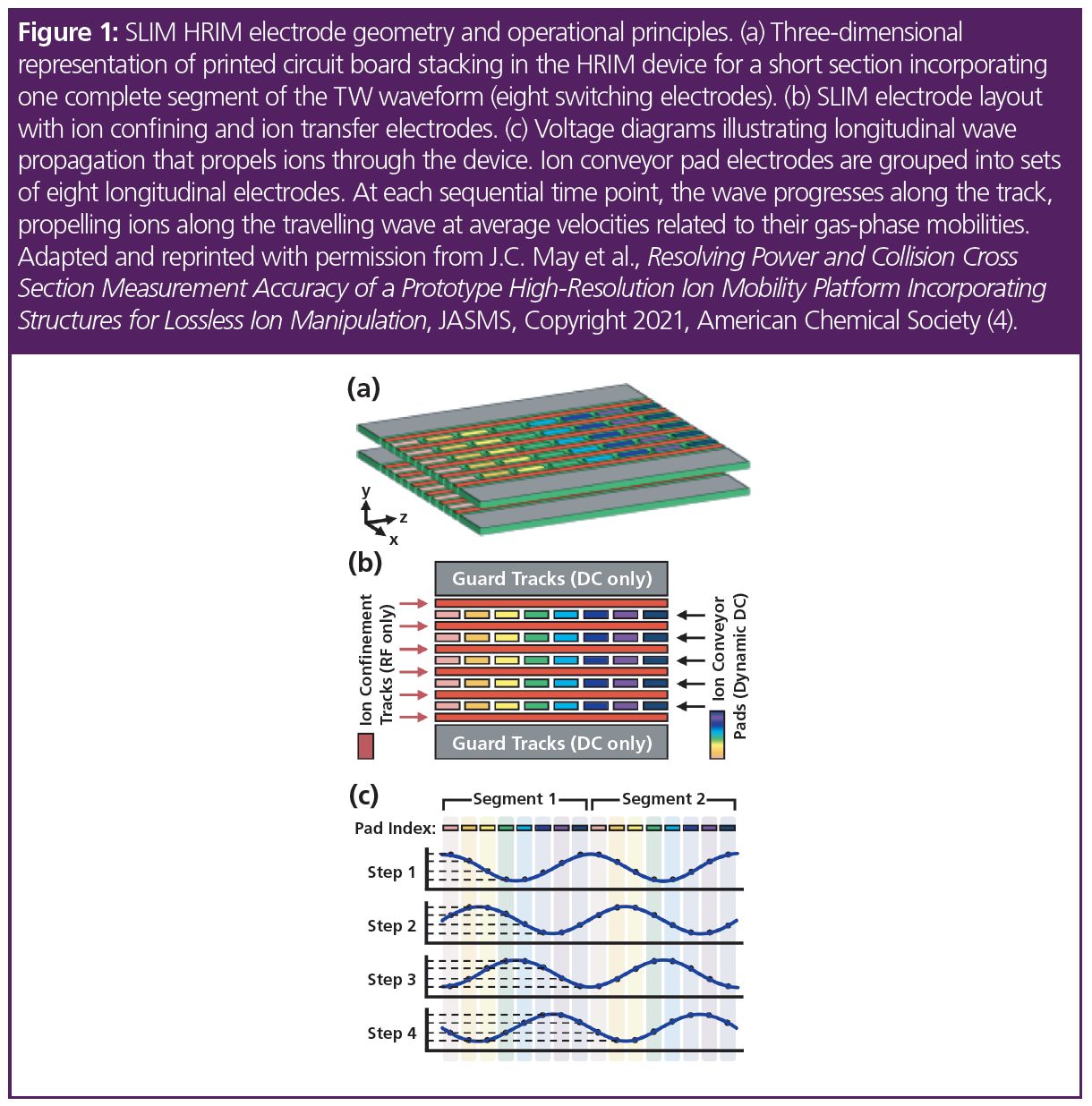
Experimental
Data were acquired using a high-resolution ion mobility spectrometer (Mobie, Mobilion Systems) integrated with a commercial quadrupole time-of-flight mass spectrometer (6545, Agilent Technologies). An LC system (1290 Infinity II, Agilent) was used to introduce samples to the HRIM-MS system via flow injection analysis (20 µL injection volume; 100 µL/min flow rate). Samples were ionized via electrospray (Jet Stream, Agilent) operated at 4.0 kV on the entrance capillary and 2.0 kV on the focusing nozzle lens. Ions were transferred to vacuum via a resistive glass capillary and collected by a source ion funnel (200 Vpp, 1.1 MHz, 1–10 Torr), where they were focused radially and introduced to the HRIM PCB stack. The HRIM separation region utilized ~2.5 Torr of high purity nitrogen gas, which was metered by a gas flow controller (Alicat Scientific) monitored with a capacitance gauge (627F Baratron, MKS Instruments), which provided a regulation of better than ±0.002 Torr.
Results and Discussion
HRIM resolving power (CCS/ΔCCS) was found to be greater than 200 across a range of ion masses and instrument parameters (travelling wave speeds and amplitudes). In many cases, resolving powers greater than 300 were achieved, most notably when analytes were measured at the threshold between nonselective “ion surfing” to mobility‑selective ion drift, which corresponds to ion speeds approximately 30–70% greater than the travelling wave speed.
The ability of HRIM to resolve analytes that are isobaric in mass was evaluated using a series of isomers from different biomolecular classes (peptides, lipids, and carbohydrates), including reversed sequence peptides (SDGRG and GRGDS), triglyceride double bond positional isomers (TG 3,6,9 and TG 6,9,12), trisaccharides (melezitose, raffinose, isomaltotriose, and maltotriose), and ganglioside lipids (GD1a/GD1b). HRIM was able to resolve the corresponding isomeric mixtures (Figure 2), which were found to be unresolvable using the standard resolution drift tube IM instrument (see the middle row of Figure 2) (4).
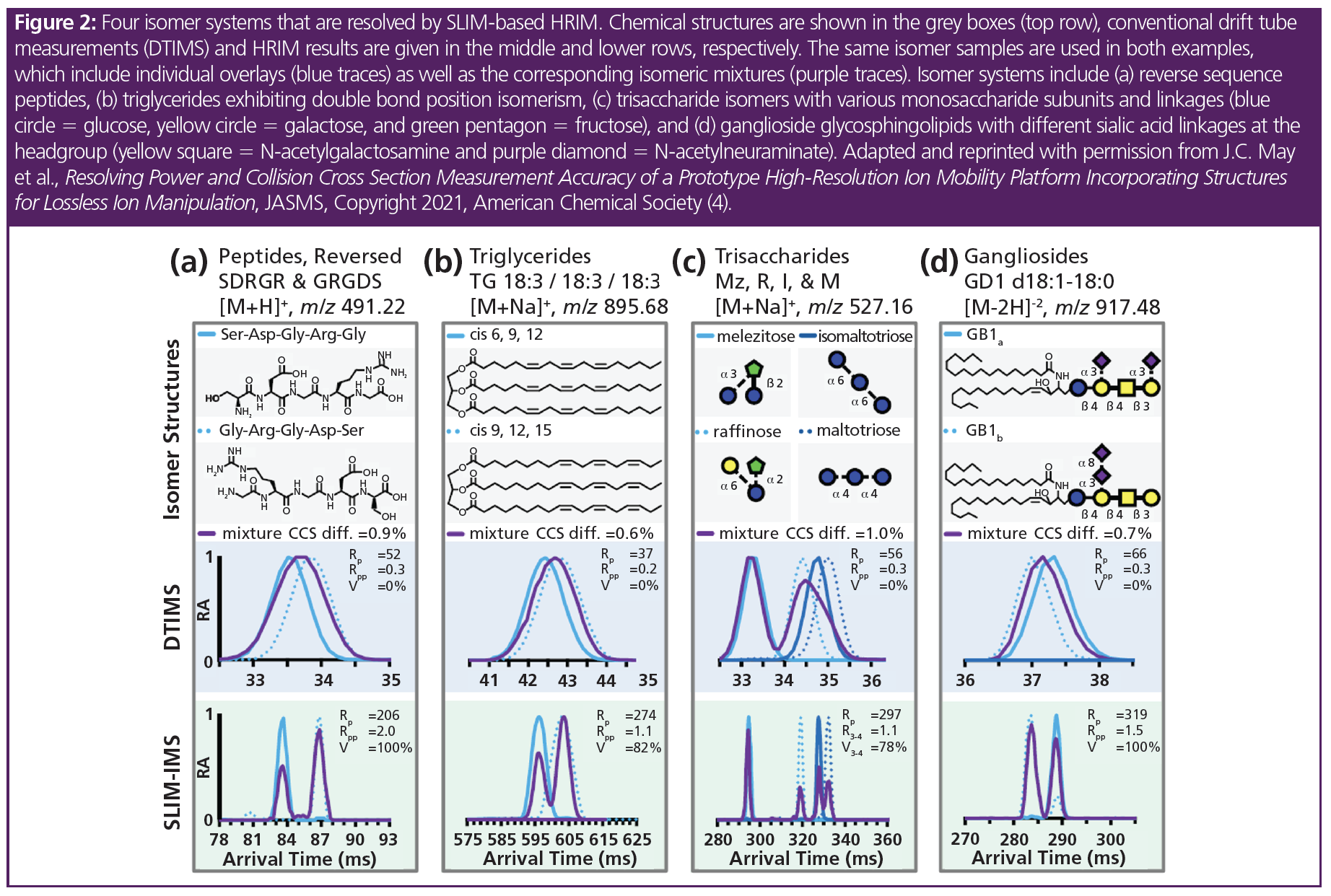
These results indicated that HRIM-MS can resolve peaks with CCS differences as small as ~0.6% without the need for targeting a specific separation window or extending the analysis time. Furthermore, under these high-resolution conditions, the measured CCS values were found to exhibit low biases (<0.5%) compared to CCS measurements obtained on a conventional drift tube instrument. All ions were separated by HRIM in less than one second, which was approximately three orders of magnitude faster than LC. Importantly, all analytes could access high-resolution conditions (>200) within the same HRIM spectrum simultaneously, which indicated that HRIM was well‑suited for untargeted, discovery applications.
Conclusion
HRIM-MS utilizing SLIM to enable the transfer and mobility separation of ions across a large distance (~13 m) was critically evaluated in terms of upper resolution and the ability to resolve isomeric mixtures. The HRIM resolving power (CCS/ΔCCS) was benchmarked between 230 and 315 for a commonly used MS tuning mixture. Notably, all analytes within this mixture were transmitted within a short dispersion time frame (<700 ms) and all exhibited resolving powers in excess of 230, indicating that HRIM-MS was well‑suited for high‑throughput broadband separations, which are important analytical figures‑of‑merit for untargeted studies. For the separations of several biochemical isomers (peptides, lipids, and carbohydrates), HRIM-MS achieved near or full baseline resolution for the corresponding isomeric mixtures, with measured peak spacings as little as 0.6% difference in CCS.
As a result, it was concluded that HRIM provided improved resolution over conventional IM without trade‑offs, meaning that HRIM-MS not only increased throughput but also provided enhanced separation capabilities and additional structural information in a shorter time, with an easily transferable method. Because HRIM methods are analyte-agnostic, the benefits of speed, reproducibility, and resolution can be applied to assays of different biomolecule classes—proteins, peptides, lipids, glycans, and other metabolites—without having to change out hardware or perform extensive method development to achieve optimal results. Compared to other ion mobility separation techniques, HRIM-MS provided high resolution separation across a broad m/z range.
HRIM-MS provides fast, efficient, high‑resolution critical quality attribute analysis of biologic therapeutics—capabilities beneficial for scientists working with complex and challenging analyte classes, such as glycans, peptides, and lipids.
References
- J.C. May and J.A. McLean, Anal. Chem. 87(3), 1422–1436 (2015).
- K. Wongtrakul-Kish, I. Walsh, L.C. Sim, A. Mak, B. Liau, V. Ding, N. Hayati, H. Wang, A. Choo, P.M. Rudd, and T. Nguyen-Khuong, Anal. Chem. 91(14), 9078–9085 (2019).
- A.L. Hollerbach, A. Li, A. Prabhakaran, G. Nagy, C.P. Harrilal, C.R. Conant, R.V. Norheim, C.E. Schimelfenig, G.A. Anderson, S.V. Garimella, and R.D. Smith, Anal. Chem. 92(11), 7972–7979 (2020).
- J.C. May, K.L. Leaptrot, B.S. Rose, K.L. Wormwood Moser, L. Deng, L. Maxon, D. DeBord, and J.A. McLean, J. Am. Soc. Mass Spectrom. 32(4), 1126–1137 (2021).
Jody C. May is Research Assistant Professor at Vanderbilt University. He received his B.S. in chemistry from the University of Central Arkansas in 2001 and his Ph.D. in analytical chemistry from Texas A&M University in 2009. Jody held a postdoctoral position at Vanderbilt University before transitioning into a research faculty position in 2012. Jody’s research interests intersect multidimensional mass spectrometry techniques with advanced informatics to elucidate unseen and undiscovered chemical space.
John A. McLean is Stevenson Professor of Chemistry and Chair at Vanderbilt University; he is also the Director of the Vanderbilt Center for Innovative Technology. He received his B.S. in chemistry from the University of Michigan and his Ph.D. from the George Washington University. Following postdoctoral training at Forschungszentrum Jülich in Germany, and at Texas A&M University, he joined the Vanderbilt faculty in 2006.
Daniel DeBord is Vice President of R&D at MobilIion Systems. Daniel has extensive experience in the field of mass spectrometry. After receiving his Ph.D. in analytical chemistry from Texas A&M University, Daniel served as Associate Director of the Advanced Mass Spectrometry Facility at Florida International University. His industry experience includes positions at BASF and 1st Detect Corporation, where he worked to design and develop miniaturized MS-based detection systems for a variety of markets.
Katrina L. Leaptrot is Research Assistant Professor in the Vanderbilt University Chemistry Department. She received her B.S. in biology and chemistry from the University of Charleston (West Virginia) in 2011 and her Ph.D. in chemistry from Vanderbilt University in 2018. Following postdoctoral training at Vanderbilt University, she transitioned into a research faculty position in 2020 for the McLean Research Group, the Vanderbilt Center for Innovative Technology, and the SyBBURE Searle Undergraduate Research Programme. Katrina’s research focuses on analyses of lipids and related biomolecules via liquid chromatography–ion mobility‑mass spectrometry.
Bailey S. Rose is a recent graduate from the McLean Research Group at Vanderbilt University with a Ph.D. in chemistry. She received her B.S. in chemistry from Belmont University in 2017. Bailey’s research focuses on the application of ion mobility‑mass spectrometry and novel informatics approaches to untargeted metabolomics and lipidomics in support of high-confidence molecular annotation.
LiuLin Deng is Principal Scientist at Mobilion Systems Inc. and is currently responsible for designing, constructing, validating, and developing the next generation ion mobility spectrometry based upon SLIM technology.
Kelly Wormwood Moser is leading Mobilion’s application development, working with industry collaborators to develop methods to demonstrate HRIM separation capabilities with biological samples. Kelly is leveraging her Ph.D. research experience, where, under the direction of Dr. Costel Darie, she used mass spectrometry to identify potential protein biomarker signatures for autism spectrum disorders.
E-mail: jody.c.may@vanderbilt.edu
Website: www.mobilionsystems.com

Altering Capillary Gas Chromatography Systems Using Silicon Pneumatic Microvalves
May 5th 2025Many multi-column gas chromatography systems use two-position multi-port switching valves, which can suffer from delays in valve switching. Shimadzu researchers aimed to create a new sampling and switching module for these systems.
Studying Cyclodextrins with UHPLC-MS/MS
May 5th 2025Saba Aslani from the University of Texas at Arlington spoke to LCGC International about a collaborative project with Northwestern University, the University of Hong Kong, and BioTools, Inc., investigating mirror-image cyclodextrins using ultra-high performance liquid chromatography–tandem mass spectrometry (UHPLC–MS/MS) and vibrational circular dichroism (VCD).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)
















