IC–ICP-MS Analysis of Gadolinium-Based MRI Contrast Agents
Metrohm Application Note
The use of contrast agents in non-invasive diagnostics provides a specific and high-detailed picture of organs and makes it possible to differentiate between healthy and diseased tissue. Whilst radiocontrast agents (RCA) change the X-ray absorption of the radiographed organ relative to its surrounding tissue, contrast agents for magnetic resonance imaging (MRI) are based on the use of magnetic fields caused by charged elementary particles such as protons and electrons. Compared to traditional X-ray imaging, MRI uses no ionizing radiation. MRI is based on the same principles as NMR spectroscopy and thus takes advantage of the absorption and emission of energy in the radio frequency range.
The nucleus of the hydrogen atom, the proton, is of particular importance in MRI because of the magnetic momentum caused by its intrinsic angular momentum (spin) and the frequency with which it occurs in water, fat or protein molecules in the organism. A short, high-frequency radio pulse changes the spin orientation of the protons before the spins — after the signal is switched off — release the previously absorbed energy again and return to the initial state. This phenomenon is termed relaxation. The signals emitted are recorded by highly-sensitive detector coils and are shown in high-contrast cross sections.
Paramagnetic contrast agents, which have unpaired electrons in the outer electron shell and thus also a magnetic momentum, are used to improve imaging. Electrons affect the protons since they have a 657-times stronger magnetic momentum than protons. As a result, both the proton density in the tissue is changed directly and the local magnetic field is changed indirectly by the interaction between the electron spin of the contrast agent and the surrounding hydrogen nuclei.
Apart from the paramagnetic elements manganese and iron, the lanthanide gadolinium, with its seven unpaired electrons in the outer electron shell, is one of the metals most commonly used in MRI contrast agents. Because of its intrinsic toxicity, however, gadolinium cannot be used in the free ionic (Gd3+ ), but only in the form of its water-soluble chelate complexes (Figure 1). In particular, the derivates of diethylenetriaminepentaacetic acid (DTPA) have proved useful as MRI contrast-enhancing agents. Nowadays every second MRI procedure is improved by contrast agents.
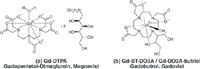
Figure 1
The gadolinium contrast agents, predominantly administered intravenously, excel in their outstanding in vivo and in vitro stability. With a half-life of approximately 2 hours, they are excreted unchanged through the kidneyurine pathway and are thus emitted into the wastewater. In municipal sewage treatment plants, where flocculation with Fe3+ or Al3+ salts is carried out to improve the settling behavior of dissolved and suspended colloidal material, the added trivalent metal ions compete with Gd3+ for the organic ligands. Depending on the thermodynamic complex stability, addition of the trivalent metal salts produces recomplexing (transmetallation) in the MRI contrast agents and thus releases toxic Gd3+ ions (1,2). Figure 2 shows the metal exchange in the gadolinium chelate Magnevist that takes place as part of iron flocculation.
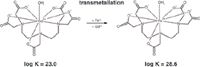
Figure 2
Using ion chromatography (IC) and subsequent inductively coupled plasma mass spectrometry (ICP-MS), the present study looks at the extent to which the Fe3+ flocculation carried out in wastewater treatment releases toxic Gd3+ ions. The impact of bench-scale flocculation is investigated for aqueous solutions of the model compounds Gadovist and Magnevist.
Experimental
IC–ICP-MS makes it possible to distinguish between different oxidation stages of metal ions (speciation) and between free and bound ions, and thus to determine the effects of the addition of Fe3+ on the stability of the gadolinium chelates. Separation of the contrast agents was carried out on a Metrosep A Supp 3 - 250/4.6 anion separation column, whereas analysis of the displaced Gd3+ ions was conducted on a Nucleosil 5 SA - 125/4.0 cation separation column using a 2-hydroxyisobutyric acid eluent. Since inline coupling of the IC with the ICP-MS was hampered by the high organic content of the eluent that caused carbon deposits on the sampler and skimmer cones, the IC fractions (4.5 mL) in a first approach were mixed with concentrated nitric acid (2.5 mL) before entering the ICP-MS. The previous acidification enhanced both the oxidation of the organic compounds and their combustion in the ICP plasma.
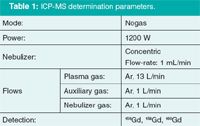
Table 1: ICP-MS determination parameters.
Aqueous solutions of Gadovist and Magnevist with a gadolinium concentration of 1 mg/L acted as MRI contrast agents. Fe3+ concentrations of 5, 10 and 20 mg/L were used to investigate the stability of the gadolinium contrast agents.
The IC–ICP-MS analysis system used consisted of an 850 Professional IC (Metrohm) and a VG PQ ExCell ICP-MS (Thermo Scientific); the ICP-MS determination parameters are shown in Table 1 and the IC parameters under the relevant chromatograms in Figures 3 and 4.

Figure 3
Results
IC–ICP-MS analysis gives two well-separated peaks for Gadovist and Magnevist on the anion-exchange column (Metrosep A Supp 3 - 250/4.0). Gadovist, as a polar, uncharged compound, elutes after only a few minutes, whilst the twice negatively charged Magnevist is stronger retained and hence elutes much later (Figure 3).
The concentrations of the competing Fe3+ and Gd3+ ions on the polyaminopolycarboxylate ligands and thus the extent of transmetallation can be determined using the Nucleosil cation separation column. The entire ion chromatographic separation was carried out by fractionation. To this end, Fe3+ was added to a Gadovist solution (Gd concentration of 1 mg/L) until a final concentration of 5 mg/L was obtained. For the released Gd3+ , the concentration profile shown in Figure 4 could be set up. Gadovist as an electrically neutral complex elutes with the solvent peak and can therefore be found in an early eluting fraction. Generally speaking, this means that an on-line IC–ICP-MS coupling allows to separate between chelated and free metal cations.

Figure 4
Figure 5 shows for the two MRI contrast agents Gadovist and Magnevist, the gadolinium displacement, which increases as Fe3+ concentration rises. This effect is more pronounced for Magnevist. According to the higher thermodynamic stability constant for the iron(III)-DTPA complex [log K = 28.6; (3)], Magnevist [log K = 23.0; (3)] can be recomplexed to approximately 80% by Fe3+ concentrations of just 5 mg/L. With an Fe3+ concentration of 20 mg/L, more than 90% is present as an iron chelate complex. In contrast, less than 10% of the Gd3+ ions are released from Gadovist — even with a higher Fe3+ supply. The crown-ether-like polyaminopolycarboxylic acid ligand arranged spherically around the gadolinium effectively shields the complex centre of the Gadovist against competing Fe3+ ions; transmetallation is largely prevented. In comparison, the linear DTPA ligands of the Magnevist only provide weak shielding of the central atom, so that gadolinium is to a large extent substituted by iron, forming the more stable iron(III) complex (4).
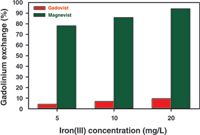
Figure 5
Conclusion
The combination of ion chromatography (IC) and inductively coupled plasma mass spectrometry (IC–ICP-MS) provides a rapid, reliable, and sensitive speciation analysis of wastewater-relevant free and chelated gadolinium compounds. IC–ICP-MS proceeds without costly sample preparation and provides important information on the supply, degradation and fate of the contrast agents in the (waste)water. The method is also highly suitable for determining compounds containing gadolinium in biological matrixes such as urine or blood.
References
1. J. Künnemeyer et al., Environ. Sci. Technol., 43, 2884–2890 (2009).
2. K. Kümmerer, Pharmaceuticals in the environment – sources, fate, effects, and risks, K. Kümmerer (Ed.), Springer Verlag, Berlin, 3rd ed., 521 pp. (2008).
3. J. Künnemeyer et al., Anal. Chem., 81, 3600–3607 (2009).
4. A. Bianchi et al., Coordination Chemistry Reviews, 204, 309–393 (2000).
Metrohm International Headquarters
Ionenstrasse, 9101 Herisau, Switzerland
tel: +41 71 353 85 04
E-mail: aw@metrohm.com Website: www.metrohm.com

Evaluating Body Odor Sampling Phases Prior to Analysis
April 23rd 2025Researchers leveraged the advantages of thermodesorption, followed by comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC/TOF-MS), to compare and assess a variety of sampling phases for body odor.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














