Analysis of Lisinopril by LC–UV Using a Core Enhanced Technology Accucore RP–MS Column
Thermo Scientific Application Note
Introduction
Thermo Scientific Accucore HPLC columns use Core Enhanced Technology to facilitate fast and high efficiency separations. The 2.6 µm diameter particles are not totally porous, but rather have a solid core and a porous outer layer. The optimized phase bonding creates a series of high coverage, robust phases. Accucore RP-MS uses an optimized alkyl chain length for more effective coverage of the silica surface. This coverage results in a significant reduction in secondary interactions and thus highly efficient peaks with very low tailing. The tightly controlled 2.6 µm diameter of Accucore particles results in much lower backpressures than typically seen with sub-2 µm materials. The USP method for lisinopril specifies minimum resolution between Impurity 1 and 2-amino-4-phenylbutyric acid, between 2-amino-4-phenylbutyric acid and lisinopril, and between lisinopril and lisinopril R,S,S isomer. The maximum %RSD of the lisinopril peak area is also specified. The implementation of Accucore RP-MS in this method allowed for the lisinopril and related substances to be analyzed according to the USP monograph.
Experimental Details
Data processing: Thermo Scientific ChromQuest 5.0 Software.
Sample Preparation
A sample of Lisinopril system suitability standard was weighed and diluted in mobile phase A.
Thermo Scientific Column
Accucore RP-MS 2.6 µm, 100 × 2.1 mm (P/N 17626-102130)
Measured pressure: 159 bar
Thermo Scientific HPLC System
Column temperature: 50 °C
Injection volume: 5 µL (partial loop)
Flow rate: 0.4 mL/min
UV detection: 210 nm (data rate 10 Hz, rise time 0.1 s)
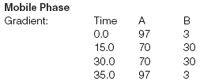
A: 0.026M monobasic sodium phosphate pH 3.75 with phosphoric acid
B: 80% mobile phase A / 20% acetonitrile
Consumables
Fisher Scientific HPLC grade water (P/N W/0106/17)
Fisher Scientific HPLC grade acetonitrile (P/N A/0626/17)
NSC Mass Spec Certified 2 mL clear vial with blue bonded PTFE silicone cap (P/N MSCERT4000-34W)
Unifilter Direct Connect Holder (3.0/2.1 mm ID) (P/N 27000)

Figure 1: Chromatogram of 1000 µg/mL of Lisinopril. (1) impurity (2) 2-amino-4-phenylbutyric acid (3) lisinopril (4) lisinopril R,S,S isomer.
Results
The original USP analytical conditions, based on a L1 250 × 460 mm, 5 µm column were scaled down using our method transfer calculator to accommodate for the column geometry reduction. The analysis was carried out on an Accucore RP-MS 2.6 µm, 100 × 2.1 mm column. The pH was also lowered to take advantage of the increased level of deactivation of the Accucore material compared to the material originally used to develop the method. The requirements for the USP method were achieved as demonstrated in Table 1.
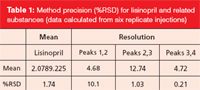
Table 1: Method precision (%RSD) for lisinopril and related substances (data calculated from six replicate injections)
Conclusions
The use of Accucore RP-MS column allowed to successfully scale down the USP method for the analysis of lisinopril and related substances, in order to increase sample throughput. The analytical results exceeded the specifications stated in the USP monograph. There was also excellent reproducibility between runs. Accucore RP-MS columns are therefore an excellent choice for the fast analysis of lisinopril.
References
(1) http://www.hplctransfer.com
(2) USP Monograph for linisopril
Tudor Road, Manor Park, Runcorn, Cheshire WA7 1TA, UK
tel: +44 (0) 1928 534110

Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.















