Validation of a Method for the Separation of Ziprasidone and its Degradants Using Empower 2 with Method Validation Manager
Waters Application Note
Introduction
The process of running a validation study, from defining tests, specifications and acceptance criteria to sample testing and analysis, is a data intensive and time consuming process. The use of additional spreadsheets and paper print-outs to process data and track results is an error-prone process that can be remedied using a validation software package such as Empower 2 with Method Validation Manager (MVM). In this application, the validation of a method for the separation of ziprasidone and impurities from a forced degradation is demonstrated using Empower 2 with MVM. Intermediate precision was also tested using a Method Validation Kit, to ensure reliability of the method across three different batches of the same column chemistry. The use of efficient sample set designs, along with automated data processing using Empower 2 with MVM, allowed six validation tests to be performed and processed in one day with simple tracking of the validation study.
Results and Discussion
Accuracy, linearity and repeatability tests were initially set up in MVM by specifying acceptance criteria in the validation protocol. Next, one sample set was written to generate data for all three tests, enabling MVM to use the results from one sample set run to complete testing for accuracy, linearity and repeatability. Validation results are shown in Table 1. The use of efficient sample sets greatly minimizes sample preparation and instrument time. Automated data processing using Empower 2 with MVM allowed rapid determination of the status and results of each test, without the need to export data to a separate spreadsheet for manual analysis. The intermediate precision of the method was evaluated across column batches using a Method Validation Kit (MVK), which provide three different batches of the same column to assess the analytical variability that might be seen using multiple batches of columns (Figure 1). The use of MVKs in both method development and validation stages can promote analytical ruggedness as columns are replaced over the lifetime of the assay.
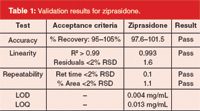
Table 1: Validation results for ziprasidone.
Conclusions
Software-based validation approaches such as Empower 2 with MVM use efficient sample sets and experimental designs, allowing validation testing to be performed much faster than setting up and running one experiment at a time. MVM requires no additional software to validate, and eliminates the need for manual analysis, greatly reducing transcription error. All samples are run, processed and compiled in one location, facilitating data tracking, improving data security and increasing audit confidence. Intermediate precision testing using MVKs help ensure long term ruggedness of the method across three different batches of columns, reducing the risk of reproducibility issues and downstream re-validation on a method developed using only one column or one batch of packing material.

Figure 1: Reproducibility of the ziprasidone peroxide degradation separation on three different batches of ACQUITY UPLC CSH C18 using a method validation kit.
For full application, go to www.waters.com/ziprasidone
©2011 Waters Corporation. Waters, The Science of What's Possible, ACQUITY UPLC and Empower are trademarks of Waters Corporation.
Waters Corporation
34 Maple Street, Milford, Massachusetts 01757, USA
tel: +1 (508) 478-2000 fax: +1 (508) 478-1990
Website: http://www.waters.com

Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.















